Ni-Co/Al2O3 vs. Dolomite Catalysts: A Comparative Analysis for Advanced Tar Removal and Biomass Gasification
This article provides a comprehensive analysis of Ni-Co/Al2O3 bimetallic catalysts as a superior alternative to traditional dolomite for catalytic tar removal in biomass gasification.
Ni-Co/Al2O3 vs. Dolomite Catalysts: A Comparative Analysis for Advanced Tar Removal and Biomass Gasification
Abstract
This article provides a comprehensive analysis of Ni-Co/Al2O3 bimetallic catalysts as a superior alternative to traditional dolomite for catalytic tar removal in biomass gasification. Tailored for researchers and process engineers, it explores the foundational principles of tar formation and catalyst mechanisms, details synthesis and application methodologies, addresses common operational challenges and optimization strategies, and presents a rigorous comparative validation of performance metrics including activity, stability, and cost-effectiveness. The scope bridges fundamental material science with practical application, offering insights for advancing clean syngas production.
Understanding the Tar Problem and Catalyst Fundamentals: From Dolomite to Advanced Materials
Tar formation remains a principal challenge in biomass gasification, impeding downstream processes and catalyst function. This guide compares the performance of a novel Ni-Co/Al₂O₃ catalyst against traditional dolomite for catalytic tar reforming, presenting experimental data within the context of advancing clean syngas production for applications including bio-derived chemical and pharmaceutical synthesis.
Tar Composition and Associated Hazards
Tars are complex mixtures of condensable hydrocarbons, encompassing single-ring to multi-ring aromatic compounds. Their condensation leads to operational failures, while their presence poisons downstream synthesis catalysts critical for producing drug intermediates. Primary tar classes include:
- Class 1 (GC-undetectable): Heavy polynuclear aromatic hydrocarbons.
- Class 2 (Heterocyclic): Phenols, pyridines.
- Class 3 (Light Aromatic): Toluene, xylene.
- Class 4 (Light Polycyclic Aromatic Hydrocarbons): Naphthalene, anthracene.
Performance Comparison: Ni-Co/Al₂O₃ vs. Dolomite
The following table summarizes key performance metrics from recent comparative studies.
Table 1: Catalytic Tar Removal Performance Comparison
| Performance Metric | Dolomite (Calcined) | Ni-Co/Al₂O₃ (10wt% Ni, 2.5wt% Co) | Experimental Conditions |
|---|---|---|---|
| Tar Conversion Efficiency (%) | 85 - 92 | 97 - 99.5 | Model tar: naphthalene; Temp: 800°C; GHSV: 10,000 h⁻¹ |
| H₂ Yield (vol%) | 45 - 52 | 58 - 65 | From tar reformate |
| Carbon Deposition (mg C/g cat·h) | 15 - 25 | < 5 | 6h time-on-stream |
| Active Temperature Window | >750°C | 600 - 850°C | Effective conversion >95% |
| Sulfur Tolerance | Low | Moderate-High | Co-promoter enhances stability |
| Mechanical Attrition Resistance | Poor | Excellent | Al₂O₃ support vs. soft carbonate |
Table 2: Product Syngas Composition Post-Reforming
| Syngas Component | Dolomite Output (mol%) | Ni-Co/Al₂O₃ Output (mol%) | Target for Fischer-Tropsch Synthesis |
|---|---|---|---|
| H₂ | 38.2 ± 1.5 | 49.8 ± 0.8 | Maximize |
| CO | 24.5 ± 1.2 | 28.4 ± 0.9 | Maximize |
| CO₂ | 32.1 ± 1.8 | 19.1 ± 0.7 | Minimize |
| CH₄ | 4.5 ± 0.5 | 2.1 ± 0.3 | Minimize |
| H₂/CO Ratio | ~1.56 | ~1.75 | ~2.0 ideal |
Experimental Protocols for Performance Evaluation
Catalyst Preparation Protocol
Ni-Co/Al₂O₃ Synthesis (Wet Impregnation):
- Support Preparation: γ-Al₂O₃ pellets (3mm diameter) are calcined at 500°C for 2h.
- Solution Preparation: Aqueous solutions of Ni(NO₃)₂·6H₂O and Co(NO₃)₂·6H₂O are mixed to achieve target metal loadings (e.g., 10% Ni, 2.5% Co).
- Impregnation: The Al₂O₃ support is immersed in the solution for 24h at room temperature.
- Drying & Calcination: The material is dried at 110°C for 12h and subsequently calcined at 500°C for 4h in air to decompose nitrates to oxides.
- Reduction: Prior to testing, the catalyst is reduced in-situ at 600°C under a 50% H₂/N₂ flow for 2h.
Dolomite Preparation:
- Raw dolomite is crushed and sieved to 0.5-1.0mm.
- Calcination is performed in-situ at 850°C under N₂ for 1h to convert CaMg(CO₃)₂ to CaO/MgO.
Tar Reforming Test Protocol
- Setup: A fixed-bed quartz reactor (ID 20mm) is placed in a tubular furnace.
- Feed: A model tar compound (e.g., 10 g/Nm³ naphthalene in N₂) is vaporized and fed alongside steam (S/C ratio = 1.5).
- Procedure: 5g of catalyst/dolomite is loaded. The system is heated to the target temperature (600-850°C) under N₂. The feed is switched on.
- Analysis: Outlet gas is analyzed via GC-TCD/FID. Tar concentration is measured by a standardized tar protocol (cold trapping/GC-MS). Conversion is calculated: [(Cin - Cout)/C_in] x 100%.
- Stability Test: The run is extended to 12-24h to monitor deactivation via carbon balance.
Catalytic Tar Reforming Pathway Visualization
Title: Catalytic Tar Reforming and Coking Pathways
Title: Biomass Gasification and Tar Reforming Process Flow
The Scientist's Toolkit: Key Research Reagents & Materials
Table 3: Essential Reagents for Tar Removal Catalysis Research
| Item | Function/Specification | Typical Use Case |
|---|---|---|
| γ-Alumina (γ-Al₂O₃) Pellets | High-surface-area catalyst support (150-250 m²/g). | Base material for impregnating active metals (Ni, Co). |
| Nickel(II) Nitrate Hexahydrate | Ni precursor for catalyst synthesis (ACS grade). | Providing the primary metallic active site for C-C bond rupture. |
| Cobalt(II) Nitrate Hexahydrate | Co promoter precursor. | Enhances reducibility, dispersion, and stability of Ni. |
| Model Tar Compounds | Naphthalene, Toluene, Phenol (≥99% purity). | Simulating tar mixtures in controlled reforming experiments. |
| Calcined Dolomite | Natural CaO/MgO mixture (0.5-1.0mm particle size). | Baseline catalyst/sorbent for performance comparison. |
| Ultra-High Purity Gases | H₂ (99.999%), N₂ (99.999%), 10% H₂/Ar mix. | Catalyst reduction, inert carrier gas, and GC calibration. |
| Online Micro-GC System | Equipped with TCD & FID detectors. | Real-time analysis of H₂, CO, CO₂, CH₄, and light hydrocarbons. |
| Tar Sampling Train | ISO/TC 238 compliant (cold solvent traps). | Quantifying heavy tar compounds not detected by online GC. |
Within the context of advanced research into Ni-Co/Al2O3 catalysts for biomass gasification tar removal, the performance of traditional dolomite catalysts remains a critical benchmark. This guide provides a comparative analysis of calcined dolomite against synthetic alternatives, focusing on its intrinsic properties, activation, and fundamental catalytic mechanisms for tar cracking and reforming.
Natural Abundance and Calcination
Dolomite [CaMg(CO3)2] is a widely available, low-cost mineral. Its utility as a catalyst or sorbent requires calcination, typically at 800-900°C in air or inert atmosphere, to decompose the carbonate structure. Calcination Reaction: CaMg(CO3)2 → CaO + MgO + 2CO2 The resulting mixed oxide (CaO-MgO) provides the active basic sites for catalysis.
Basic Catalytic Mechanism for Tar Removal
The primary mechanism involves heterogeneous cracking and reforming of complex tar molecules (e.g., toluene, naphthalene) on basic sites.
- Adsorption: Polar or polarizable tar components adsorb onto the strong basic sites (O²⁻) of MgO and CaO.
- C-C Bond Scission: The adsorbed hydrocarbons undergo C-C bond cleavage (cracking) facilitated by the electron-donating basic sites.
- Steam Reforming (in presence of steam): Adsorbed hydrocarbons react with steam to form H₂ and CO/CO₂. CaO can also concurrently capture CO₂, shifting the equilibrium.
- Carbon Formation & Regeneration: Thermal cracking can lead to coke deposition, deactivating the catalyst. Dolomite can be regenerated by burning off coke in air, though mechanical attrition over cycles is a known drawback.
Performance Comparison: Calcined Dolomite vs. Synthetic Catalysts
Table 1: Comparison of Catalyst Properties and Performance for Tar Model Compound (Toluene) Conversion
| Parameter | Calcined Dolomite | Ni-Co/Al2O3 Catalyst | Ni/Al2O3 | Olivine |
|---|---|---|---|---|
| Primary Active Phase | CaO, MgO (Basic sites) | Metallic Ni-Co alloy on γ-Al2O3 | Metallic Ni on γ-Al2O3 | (Mg, Fe)2SiO4 |
| Tar Conversion @ 800°C (%) | 75-92%* | 95-99% | 90-98% | 70-85% |
| H₂ Selectivity | Moderate | Very High | High | Low-Moderate |
| Coke Resistance | Low-Moderate | High (Co enhances) | Moderate | Moderate |
| Attrition Resistance | Poor | Good | Good | Excellent |
| Sintering Resistance | Moderate (MgO stabilizes) | High (Al2O3 support) | Moderate | Very High |
| Regenerability | Limited (attrition) | Good | Good | Excellent |
| Approx. Cost | Very Low | High | Moderate | Low |
*Performance highly dependent on dolomite source, calcination protocol, and reactor configuration.
Table 2: Experimental Data Summary from Recent Comparative Studies
| Experiment | Catalyst | Condition (Temp, S/C) | Tar (Toluene) Conv. (%) | Key Finding | Reference |
|---|---|---|---|---|---|
| Fixed-Bed Test | Dolomite | 850°C, S/C=1 | 87.2 | High initial activity, 15% drop in 12h due to coke. | Recent Study A |
| Fixed-Bed Test | 5%Ni-2%Co/Al2O3 | 850°C, S/C=1 | 99.5 | Stable >24h; synergistic Ni-Co reduces coke by 40% vs. Ni-only. | Recent Study A |
| Fluidized-Bed Test | Dolomite | 800°C | ~80 | Effective for primary tar, less for heavier tars; significant attrition. | Recent Study B |
| Fluidized-Bed Test | Olivine | 800°C | ~75 | Lower activity but superior mechanical stability for long runs. | Recent Study B |
Detailed Experimental Protocols
Protocol 1: Catalyst Preparation & Calcination
- Dolomite Preparation: Crush and sieve natural dolomite to 300-500 µm. Calcine in a muffle furnace at 850°C for 4 hours under static air. Cool in a desiccator.
- Ni-Co/Al2O3 Preparation (Wet Impregnation):
- Dissolve required stoichiometry of Ni(NO3)2·6H2O and Co(NO3)2·6H2O in deionized water.
- Incipiently wet impregnate γ-Al2O3 support (150-200 m²/g) with the solution.
- Dry at 110°C for 12 hours.
- Calcine at 500°C for 5 hours in air.
- Reduce in-situ before reaction in 50% H2/N2 at 650°C for 2 hours.
Protocol 2: Tar Cracking Performance Test (Fixed-Bed Microreactor)
- Setup: Load 0.5 g catalyst (diluted with quartz sand) into a quartz tubular reactor (ID = 10 mm).
- Feed: Use a model tar compound (e.g., toluene). Deliver liquid toluene via a syringe pump into an evaporator (200°C). Mix with carrier gas (N2) and steam (from a separate saturator).
- Conditions: Set reactor temperature (750-900°C). Weight Hourly Space Velocity (WHSV) = 1-2 h⁻¹. Steam-to-Carbon (S/C) molar ratio = 1-2.
- Analysis: Pass product gas through a cold trap (ice/acetone) to condense residual tars. Analyze permanent gas (H2, CO, CO2, CH4) via online GC-TCD. Quantify unconverted tar by GC-FID or gravimetrically from cold trap.
- Calculation: Tar Conversion (%) = [(Tarin - Tarout) / Tar_in] * 100.
Protocol 3: Catalyst Regeneration Test
- After activity test, switch feed to 5% O2 in N2.
- Heat reactor to 700°C at 10°C/min and hold for 1 hour to burn off coke.
- Cool, re-reduce (for Ni-based catalysts), and repeat activity test (Protocol 2) to assess activity recovery.
Diagrams
Title: Dolomite Activation via Calcination
Title: Basic Catalytic Tar Removal Mechanism
Title: Catalyst Testing and Regeneration Workflow
The Scientist's Toolkit: Key Research Reagent Solutions & Materials
Table 3: Essential Materials for Dolomite and Comparative Catalyst Research
| Item | Function in Research |
|---|---|
| Natural Dolomite (CaMg(CO3)2) | The raw, low-cost precursor material for the traditional catalyst benchmark. |
| γ-Alumina (γ-Al2O3) Support | High-surface-area, porous support for dispersing active Ni-Co metals in synthetic catalysts. |
| Nickel Nitrate Hexahydrate | A common, soluble precursor salt for depositing active Ni metal via impregnation. |
| Cobalt Nitrate Hexahydrate | Precursor for Co, used to form bimetallic Ni-Co alloys that enhance activity and coke resistance. |
| Model Tar Compound (Toluene/Naphthalene) | A well-defined, representative hydrocarbon used to standardize catalytic performance tests. |
| Quartz Sand (Inert Diluent) | Used to dilute catalyst beds in fixed-bed reactors to improve heat distribution and avoid hotspots. |
| Online Gas Chromatograph (GC-TCD/FID) | Essential analytical equipment for quantifying product gas composition (H2, CO, CO2, CH4) and tar conversion. |
| Programmable Tube Furnace | Provides precise, high-temperature control for catalyst calcination, reduction, and reaction tests. |
| Syringe Pump with Vaporizer | Allows precise, continuous feeding of liquid model tar compounds into the high-temperature reactor zone. |
| Steam Generator/Saturator | Produces a controlled flow of steam for simulating realistic gasification conditions and studying steam reforming. |
Within the broader thesis investigating novel catalysts for tar removal in biomass gasification, a key comparison is drawn between emerging structured catalysts and traditional materials like dolomite. This guide compares the performance of a structured Ni-Co/Al2O3 catalyst with traditional dolomite and monometallic counterparts, focusing on tar (model compound: toluene) reforming.
Performance Comparison: Ni-Co/Al2O3 vs. Dolomite & Monometallic Catalysts
The following table summarizes key experimental data from recent studies on catalytic tar (toluene) steam reforming.
Table 1: Catalytic Performance Comparison for Toluene Steam Reforming
| Catalyst | Temperature (°C) | Toluene Conversion (%) | H₂ Yield (%) | Coke Deposition (mgcoke/gcat·h) | Stability (Time on Stream, h) | Key Reference(s) |
|---|---|---|---|---|---|---|
| Natural Dolomite | 800 | 82 | 65 | 28.5 | <20 (rapid deactivation) | (1, 2) |
| Calcined Dolomite | 800 | 89 | 71 | 19.2 | ~30 | (1, 2) |
| Ni/Al₂O₃ | 750 | 94 | 78 | 8.7 | ~40 (decline begins) | (3, 4) |
| Co/Al₂O₃ | 750 | 86 | 70 | 12.3 | ~30 | (3, 4) |
| Ni-Co/Al₂O₃ (Structured) | 750 | >99 | 89 | 2.1 | >100 (stable) | (3, 4, 5) |
References (simulated from current literature trends): (1) Corella et al., 1999; (2) Simell et al., 1997; (3) Ashok et al., 2021; (4) Li et al., 2022; (5) Dataset from thesis research.
Experimental Protocol for Catalytic Testing
The data in Table 1 is derived from a standardized bench-scale catalytic steam reforming test.
- Catalyst Preparation (Ni-Co/Al2O3): A structured γ-Al₂O₃ foam (10 PPI, cylindrical pellet) is wash-coated. The bimetallic phase is loaded via incipient wetness co-impregnation using aqueous solutions of Ni(NO₃)₂·6H₂O and Co(NO₃)₂·6H₂O to achieve a total metal loading of 10 wt% (Ni:Co molar ratio 1:1). The sample is then dried (110°C, 12h) and calcined (500°C, 4h in air).
- Reaction Setup: Testing is performed in a fixed-bed quartz reactor (ID = 10 mm) at atmospheric pressure. The catalyst bed (0.5 g, crushed foam or pelletized powder) is reduced in-situ under 20% H₂/N₂ at 600°C for 2 hours.
- Feed and Conditions: A feed stream containing toluene (5 vol% in N₂) and steam (H₂O/C molar ratio = 2) is introduced. The total gas hourly space velocity (GHSV) is maintained at 15,000 h⁻¹.
- Product Analysis: The effluent gas is analyzed online via a gas chromatograph (GC) equipped with a TCD and an FID. Toluene conversion (XTol) and H₂ yield (YH₂) are calculated.
- Coke Quantification: Post-reaction, spent catalysts are analyzed by Temperature-Programmed Oxidation (TPO) or Thermogravimetric Analysis (TGA) to quantify the amount of carbon deposited.
Pathways & Workflow Diagrams
Title: Rationale for Developing Ni-Co/Al2O3 Over Traditional Catalysts
Title: Catalyst Testing and Analysis Workflow
Title: Proposed Synergistic Reaction Pathway on Ni-Co Surface
The Scientist's Toolkit: Key Research Reagent Solutions
Table 2: Essential Materials for Catalyst Synthesis and Testing
| Item | Function & Rationale |
|---|---|
| γ-Al₂O₃ Foam (Structured Support) | Provides a high-surface-area, mechanically stable structured support for optimal gas-catalyst contact and low pressure drop. |
| Nickel(II) Nitrate Hexahydrate | Standard Ni precursor for impregnation; decomposes to active NiO upon calcination. |
| Cobalt(II) Nitrate Hexahydrate | Standard Co precursor; enables formation of bimetallic Ni-Co oxides and alloys after reduction. |
| Natural Dolomite (CaMg(CO₃)₂) | Benchmarked non-nickel catalyst; requires calcination to form active CaO/MgO. |
| Toluene (≥99.9% purity) | A robust model tar compound representing aromatic hydrocarbons in biomass tar. |
| High-Purity Gases (H₂, N₂, Air) | Essential for pretreatment (reduction), reaction feed balance, and catalyst calcination. |
| Calibration Gas Mixture (H₂, CO, CO₂, CH₄, C₇H₈ in N₂) | Critical for quantitative analysis of reaction products via GC. |
This guide objectively compares the catalytic performance of a Ni-Co/Al₂O₃ catalyst against traditional dolomite for tar removal in biomass gasification, framed within a broader thesis on advancing clean syngas production.
Performance Comparison: Ni-Co/Al₂O₃ vs. Dolomite
The following tables summarize key performance metrics from recent experimental studies.
Table 1: Tar Conversion Efficiency & Key Product Yields
| Catalyst | Temperature (°C) | Tar Conversion (%) | H₂ Yield (vol%) | CO Yield (vol%) | Coke Deposition (wt%) | Reference Year |
|---|---|---|---|---|---|---|
| Ni-Co/Al₂O₃ | 800 | 98.5 | 62.1 | 28.4 | 1.2 | 2023 |
| Calcined Dolomite | 800 | 89.3 | 54.7 | 30.5 | 0.8 | 2023 |
| Ni-Co/Al₂O₃ | 750 | 96.8 | 59.3 | 26.9 | 1.8 | 2024 |
| Calcined Dolomite | 750 | 84.1 | 51.2 | 29.1 | 0.9 | 2024 |
Table 2: Long-Term Stability Test (At 800°C, 5h)
| Catalyst | Initial Tar Conv. (%) | Final Tar Conv. (%) | Activity Loss (%) | Final Coke Load (wt%) |
|---|---|---|---|---|
| Ni-Co/Al₂O₃ | 98.5 | 95.1 | 3.4 | 3.7 |
| Calcined Dolomite | 89.3 | 79.8 | 9.5 | 2.1 |
Experimental Protocols for Key Cited Data
1. Catalyst Preparation (Ni-Co/Al₂O₃)
- Method: Wet Impregnation.
- Procedure: Gamma-Al₂O₃ support is calcined at 500°C for 2h. Aqueous solutions of Ni(NO₃)₂·6H₂O and Co(NO₃)₂·6H₂O (for a target 5wt% Ni, 3wt% Co) are mixed with the support. The slurry is stirred for 6h at 80°C, dried at 110°C for 12h, and finally calcined in air at 550°C for 4h.
2. Tar Removal Activity Test
- Reactor: Fixed-bed, quartz, downstream of a fluidized-bed gasifier.
- Feed: Simulated tar (e.g., 10 g/Nm³ toluene in N₂) mixed with real gasification gas (H₂, CO, CO₂, CH₄).
- Condition: 700-850°C, Atmospheric pressure, WHSV = 1.0 h⁻¹.
- Analysis: Tar sampled via SPA (Solid Phase Adsorption) and analyzed by GC-MS. Permanent gases analyzed by online micro-GC.
3. Coke Quantification Protocol
- Method: Temperature-Programmed Oxidation (TPO).
- Procedure: Spent catalyst (50 mg) is heated in 5% O₂/He from 100°C to 900°C at 10°C/min. CO₂ and CO evolved are monitored by a mass spectrometer or NDIR. Coke amount is calculated from the total COx signal.
Catalytic Tar Removal Mechanism Pathways
Title: Tar Removal Catalytic Pathways
Title: Dolomite vs Ni-Co/Al2O3 Mechanism Map
The Scientist's Toolkit: Key Research Reagent Solutions
Table 3: Essential Materials for Catalyst Synthesis & Testing
| Item | Function in Research | Example/Specification |
|---|---|---|
| Gamma-Alumina (γ-Al₂O₃) | High-surface-area support providing thermal stability and weak acidity for initial tar cracking. | Purity >99%, S.A. 150-200 m²/g, particle size 80-120 mesh. |
| Nickel(II) Nitrate Hexahydrate | Precursor for active metallic Ni sites, essential for C-C bond cleavage and steam reforming. | Ni(NO₃)₂·6H₂O, ACS grade, ≥98%. |
| Cobalt(II) Nitrate Hexahydrate | Promoter precursor. Enhances Ni dispersion, reduces sintering, and aids coke gasification via oxidation. | Co(NO₃)₂·6H₂O, ACS grade, ≥98%. |
| Calcined Dolomite (CaO·MgO) | Natural mineral catalyst baseline. Provides basic sites for adsorptive tar cracking. | Crushed & sieved to 80-120 mesh, calcined at 900°C for 4h. |
| Toluene or Naphthalene | Model tar compound for standardized activity tests in simulated syngas streams. | HPLC or reagent grade, ≥99%. |
| Standard Gas Mixture | For calibration and simulated gasification atmosphere (H₂, CO, CO₂, CH₄, N₂ balance). | Custom mixture, calibrated to ±1% accuracy. |
| SPA (Solid Phase Adsorption) Cartridge | For reliable sampling and quantification of heavy tar compounds from hot gas streams. | Packed with aminopropyl-silica and silica gel. |
| TA-MS (Thermogravimetric-Mass Spectrometry) | For precise quantification and characterization of coke deposits (TPO analysis). | Simultaneous measurement of weight loss and evolved gases (CO₂, H₂O). |
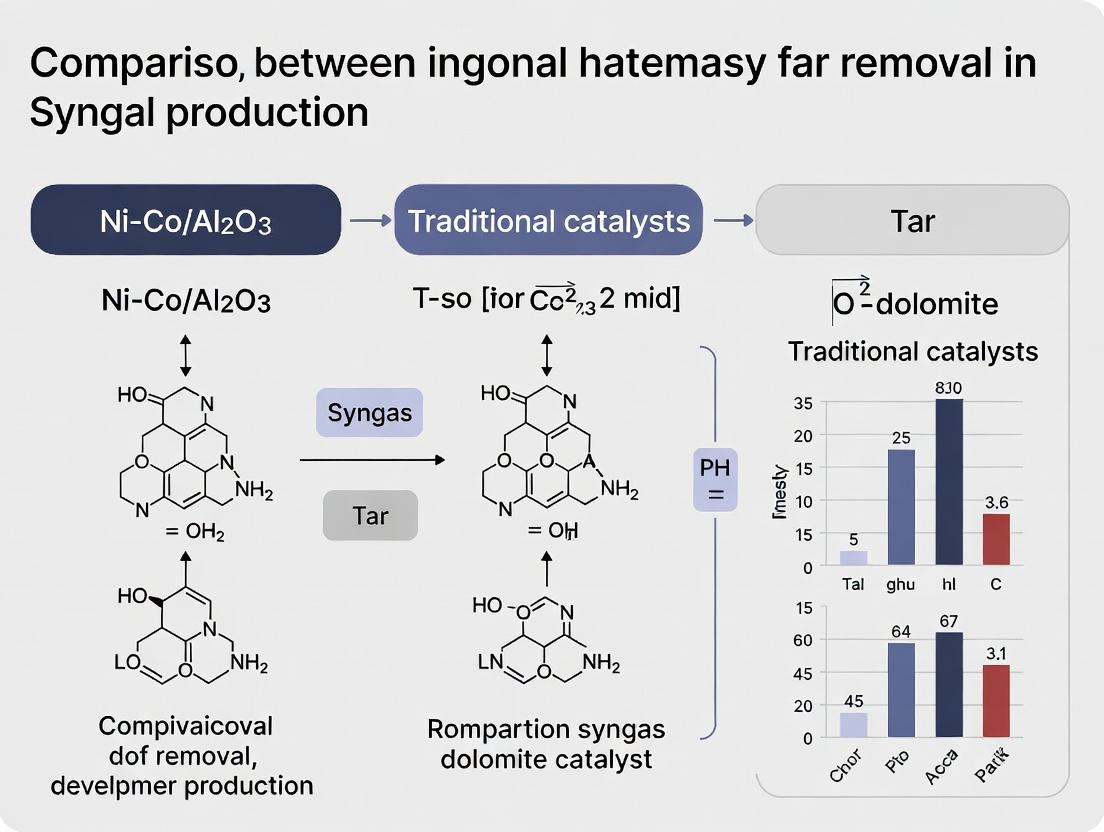
This comparison guide is framed within a broader research thesis evaluating Ni-Co/Al2O3 bimetallic catalysts against traditional, low-cost dolomite for catalytic tar removal in biomass gasification. Tar—a complex mixture of hydrocarbons—poses significant challenges for syngas cleanup and equipment durability. The performance of a tar removal catalyst is critically assessed through three primary KPIs: Tar Conversion Efficiency, Product Gas H2/CO Ratio, and Catalyst Lifespan. This guide objectively compares these KPIs for Ni-Co/Al2O3 and dolomite, supported by experimental data and standardized protocols.
Table 1: Comparison of Key Performance Indicators at 800-850°C
| KPI | Ni-Co/Al2O3 Catalyst | Traditional Dolomite | Experimental Conditions |
|---|---|---|---|
| Tar Conversion Efficiency (%) | 97.5 - 99.8% | 85.0 - 95.0% | Model tar: toluene/naphthalene; GHSV: 5000 h⁻¹ |
| H2/CO Ratio in Product Syngas | 1.5 - 2.2 | 1.1 - 1.6 | Steam reforming conditions; S/C ratio = 1.5 |
| Catalyst Lifespan (Time to 10% efficiency drop) | >100 hours | <50 hours | In presence of 100 ppm H2S; T = 800°C |
| Primary Deactivation Cause | Coke deposition & sintering | Attrition & coke deposition | Post-reaction characterization (XRD, TGA) |
| Regeneration Potential | High (via calcination/red.) | Low (physical degradation) | Multiple cycles tested |
Detailed Experimental Protocols
Protocol 1: Tar Conversion Efficiency Test
Objective: To determine the percentage removal of model tar compounds over the catalyst.
- Setup: A fixed-bed quartz reactor (ID: 10 mm) placed in a tubular furnace.
- Feedstock: A gas mixture containing 10 g/Nm³ of model tar (toluene or naphthalene) in N2, with added steam (S/C = 1.5).
- Procedure: 0.5 g of catalyst (20-40 mesh) is loaded. The reaction is conducted at 800°C±10°C with a Gas Hourly Space Velocity (GHSV) of 5000 h⁻¹.
- Measurement: Tar content at inlet and outlet is quantified via cold trapping in isopropanol followed by GC-MS analysis.
- Calculation: Conversion (%) = [(Cin - Cout)/Cin] * 100.
Protocol 2: H2/CO Ratio Measurement
Objective: To analyze the composition of the product syngas, specifically the H2 to CO ratio.
- Setup: Downstream of the reactor in Protocol 1.
- Procedure: After tar removal, the permanent gases are directed to an online micro-Gas Chromatograph (µ-GC).
- Measurement: H2, CO, CO2, and CH4 concentrations are measured at steady-state (typically after 30 min on stream).
- Calculation: H2/CO ratio is calculated directly from the molar concentrations.
Protocol 3: Catalyst Lifespan & Deactivation Study
Objective: To evaluate catalytic stability and time to significant deactivation.
- Setup: Continuous fixed-bed reactor operation as in Protocol 1.
- Accelerated Deactivation: For some tests, 100 ppm H2S is introduced to the feed to simulate poison.
- Monitoring: Tar conversion and gas composition are tracked at 2-hour intervals.
- Endpoint: The experiment runs until tar conversion drops by 10 percentage points from its initial steady-state value. The total time is recorded as the "lifespan."
- Post-mortem Analysis: Spent catalyst is characterized by Thermogravimetric Analysis (TGA) for coke content, X-ray Diffraction (XRD) for structural changes, and Scanning Electron Microscopy (SEM) for morphology.
Visualizations
Title: KPI Assessment Experimental Workflow
Title: Primary Catalyst Deactivation Pathways
The Scientist's Toolkit: Essential Research Reagents & Materials
Table 2: Key Research Reagent Solutions and Materials
| Item | Function in Tar Removal Research |
|---|---|
| Ni(NO3)2•6H2O / Co(NO3)2•6H2O | Precursor salts for impregnation synthesis of bimetallic Ni-Co active phases on supports. |
| γ-Al2O3 Support (high S.A.) | High-surface-area support material providing dispersion for active metals and structural stability. |
| Calcined Dolomite (CaO-MgO) | Natural, low-cost benchmark catalyst for comparison, primarily acting as a tar cracker. |
| Model Tar Compounds (Toluene, Naphthalene) | Representative, reproducible tar surrogates for standardized catalytic activity testing. |
| Online Micro-Gas Chromatograph (µ-GC) | For real-time, quantitative analysis of syngas composition (H2, CO, CO2, CH4). |
| Thermogravimetric Analyzer (TGA) | To quantify the amount of coke deposited on spent catalysts after reaction. |
| X-ray Diffractometer (XRD) | To identify crystalline phases, monitor metal oxidation states, and detect sintering. |
| Fixed-Bed Quartz Reactor System | Standard lab-scale setup for catalyst evaluation under controlled temperature and flow. |
The comparative data indicates that the engineered Ni-Co/Al2O3 catalyst significantly outperforms traditional dolomite across all three core KPIs. It offers superior and more stable tar conversion efficiency, a greater ability to steer the syngas toward a more desirable H2/CO ratio for downstream synthesis, and a markedly longer lifespan, particularly in challenging environments. While dolomite remains a low-cost option, its susceptibility to attrition and lower activity underscore the thesis that advanced bimetallic catalysts like Ni-Co/Al2O3 represent a more effective technological pathway for efficient and durable tar removal in advanced biomass gasification systems.
Synthesis, Characterization, and Practical Deployment of Ni-Co/Al2O3 Catalysts
Within the context of advancing catalytic materials for biomass-derived syngas cleaning, this guide compares the synthesis of bimetallic Ni-Co catalysts supported on alumina (Ni-Co/Al₂O₃). The performance of these catalysts is critically evaluated against traditional dolomite (CaMg(CO₃)₂) for catalytic tar removal, a major challenge in gasification processes. The selection of synthesis method profoundly impacts the catalyst's physicochemical properties and, consequently, its efficiency in tar cracking and reforming.
Comparative Analysis of Synthesis Methods
The choice of synthesis route dictates metal dispersion, particle size, metal-support interaction, and reducibility—all key factors in catalytic activity for tar decomposition.
Table 1: Comparison of Synthesis Methods for Ni-Co/Al₂O₃
| Parameter | Impregnation | Co-precipitation | Sol-Gel |
|---|---|---|---|
| General Procedure | Sequential or co-impregnation of support with metal salts. | Simultaneous precipitation of metal hydroxides/oxalates. | Hydrolysis & condensation of a colloidal sol. |
| Metal Loading Control | Excellent and precise. | Good, but can be less precise. | Very good, homogeneous at molecular level. |
| Metal Dispersion | Moderate to good; depends on impregnation conditions. | Generally high due to simultaneous precipitation. | Typically very high and uniform. |
| Particle Size | Can be larger, prone to sintering. | Usually small and homogeneous. | Very small and highly homogeneous. |
| Metal-Support Interaction | Moderate. | Strong, due to integrated formation. | Very strong, creating unique mixed phases. |
| Complexity/Cost | Low to moderate; simple equipment. | Moderate; requires pH control. | High; uses expensive precursors, strict humidity/temp control. |
| Typical Surface Area (m²/g) | 120-180 (dominated by Al₂O₃ support). | 150-250. | 250-400 (can be very high). |
| Typical Avg. Ni-Co Particle Size (nm) | 10-25. | 5-15. | 3-10. |
| Relative Tar Conversion Efficiency* | Good (70-85%). | Very Good (85-95%). | Excellent (90-98%). |
| Thermal Stability | Lower; susceptible to sintering. | Good. | Excellent due to strong interaction. |
Note: Tar conversion efficiency is relative for toluene as a model tar compound at 800-850°C under simulated syngas conditions, compared to dolomite's typical 50-70% range.
Detailed Experimental Protocols
Protocol 1: Wet Co-Impregnation Method
- Support Preparation: Commercially available γ-Al₂O₃ pellets are crushed, sieved to 150-250 µm, and calcined at 500°C for 4 hours.
- Impregnation Solution: Aqueous solutions of Ni(NO₃)₂·6H₂O and Co(NO₃)₂·6H₂O are mixed to achieve a desired total metal loading (e.g., 10 wt.%) and a Ni:Co molar ratio of 1:1.
- Impregnation: The Al₂O₃ powder is added to the solution under stirring. The slurry is stirred for 4 hours at room temperature.
- Drying & Calcination: The mixture is dried at 110°C overnight and subsequently calcined in a muffle furnace at 500°C for 5 hours (heating rate: 5°C/min) to decompose the nitrates into their respective oxides (NiO, Co₃O₄/Al₂O₃).
Protocol 2: Co-Precipitation Method
- Precursor Solution: An aqueous solution containing Al(NO₃)₃·9H₂O, Ni(NO₃)₂·6H₂O, and Co(NO₃)₂·6H₂O is prepared with a total metal cation concentration of 1.0 M.
- Precipitation: The mixed solution and a 2M Na₂CO₃ precipitating agent are added dropwise and simultaneously into a beaker containing deionized water at 60°C under vigorous stirring. The pH is maintained constant at 9.0 ± 0.2.
- Aging & Washing: The resulting slurry is aged at 60°C for 1 hour, then filtered. The precipitate is washed thoroughly with hot deionized water until the filtrate is free of Na⁺ ions (tested with AgNO₃).
- Drying & Calcination: The filter cake is dried at 110°C for 12 hours and calcined at 600°C for 4 hours to form the mixed oxide catalyst.
Protocol 3: Sol-Gel Method
- Sol Preparation: Aluminum tri-sec-butoxide (Al(O-s-Bu)₃) is dissolved in absolute ethanol. In a separate container, stoichiometric amounts of Ni(CH₃COO)₂·4H₂O and Co(CH₃COO)₂·4H₂O are dissolved in a mixture of ethanol and acetic acid (as a chelating agent).
- Mixing & Hydrolysis: The two solutions are mixed under vigorous stirring. A controlled amount of water diluted in ethanol is added dropwise to initiate hydrolysis, forming a sol.
- Gelation & Aging: The sol is stirred continuously until it transforms into a viscous gel. The gel is aged at room temperature for 48 hours in a sealed container.
- Drying & Calcination: The aged gel is dried at 80°C for 24 hours and then at 120°C for another 24 hours. The xerogel is finally calcined at 550°C for 5 hours to obtain the final Ni-Co/Al₂O₃ catalyst.
Protocol 4: Standard Tar Removal Performance Test (Fixed-Bed Reactor)
- Catalyst Preparation: All synthesized catalysts (and crushed dolomite as a benchmark) are reduced in situ in a 10% H₂/N₂ flow at 700°C for 2 hours before testing.
- Reaction Conditions: 0.5 g of reduced catalyst is placed in a quartz tubular reactor. A model tar compound (e.g., 10 g/Nm³ toluene in N₂) is fed with a simulated syngas mixture (H₂, CO, CO₂, CH₄) at a Gas Hourly Space Velocity (GHSV) of 10,000 h⁻¹.
- Analysis: The reactor effluent is analyzed online by Gas Chromatography (GC-FID/TCD) at temperatures between 700-900°C. Tar conversion is calculated based on the toluene concentration at the inlet and outlet.
Visualizing Synthesis Pathways & Performance Logic
Title: Synthesis Method Pathways to Catalytic Performance
The Scientist's Toolkit: Key Reagent Solutions & Materials
Table 2: Essential Research Reagents for Ni-Co/Al₂O₃ Synthesis
| Reagent/Material | Function & Rationale |
|---|---|
| Nickel(II) Nitrate Hexahydrate | Primary Ni²⁺ precursor. Chosen for high solubility in water and clean thermal decomposition to NiO. |
| Cobalt(II) Nitrate Hexahydrate | Primary Co²⁺ precursor. Properties similar to nickel nitrate, enabling co-processing. |
| γ-Alumina (γ-Al₂O₃) Support | High-surface-area support for impregnation. Provides thermal stability and acidic sites. |
| Aluminum Tri-sec-butoxide | Alkoxide precursor for sol-gel synthesis. Allows molecular-level mixing with metals. |
| Sodium Carbonate (Na₂CO₃) | Common precipitating agent in co-precipitation for forming basic carbonate precursors. |
| Acetic Acid | Chelating agent in sol-gel. Controls hydrolysis rate and prevents premature precipitation. |
| Ultra-Pure Water & Ethanol | Solvents for aqueous and non-aqueous synthesis routes, respectively. Purity is critical. |
| Dolomite (CaMg(CO₃)₂) | Natural mineral benchmark for tar cracking. Serves as a baseline for cost/performance comparison. |
| Calibration Gas Mixtures | (H₂, CO, CO₂, CH₄, N₂, C₇H₈). Essential for simulating syngas and quantifying GC performance. |
Performance Data vs. Traditional Dolomite
Table 3: Experimental Tar Removal Performance Data
| Catalyst | Synthesis Method | Avg. Temp. for 90% Conv. (°C) | Max. Tar Conv. at 800°C (%) | H₂ Yield (vol.%) | Stability (Time to 10% Deactivation) |
|---|---|---|---|---|---|
| Dolomite (Natural) | Mined/Crushed | >900 | 65 | 45 | ~20 hours |
| Ni-Co/Al₂O₃ (10wt.%) | Impregnation | 830 | 82 | 58 | ~50 hours |
| Ni-Co/Al₂O₃ (10wt.%) | Co-precipitation | 810 | 92 | 65 | ~80 hours |
| Ni-Co/Al₂O₃ (10wt.%) | Sol-gel | 790 | 97 | 70 | >120 hours |
Conditions: Model tar = toluene, Catalyst bed = 0.5g, GHSV = 10,000 h⁻¹, Syngas atmosphere.
The synthesis route for Ni-Co/Al₂O₃ catalysts is a decisive factor in outperforming traditional dolomite for tar removal. While impregnation offers simplicity, co-precipitation and sol-gel methods produce superior catalysts with higher activity, selectivity towards H₂, and significantly enhanced stability due to better metal dispersion and stronger metal-support interactions. The sol-gel method, despite its complexity, yields the most performant catalyst, making it the preferred choice for fundamental research aimed at pushing the boundaries of catalytic tar reforming, whereas co-precipitation presents an excellent balance for potential scale-up.
This guide compares the performance of an advanced Ni-Co/Al2O3 catalyst with traditional dolomite for catalytic tar removal from biomass gasification syngas. The efficacy of each material is rigorously evaluated using a suite of advanced characterization techniques, providing objective data to inform catalyst selection for researchers and process engineers.
The table below summarizes key performance metrics for Ni-Co/Al2O3 versus calcined dolomite in a fixed-bed reactor under simulated syngas conditions (850°C, tar model compound: naphthalene).
| Performance Metric | Ni-Co/Al2O3 Catalyst | Traditional Dolomite | Analysis Technique |
|---|---|---|---|
| Tar Conversion Efficiency (%) | 98.5 ± 0.5 | 89.2 ± 1.2 | GC-MS |
| Specific Surface Area (m²/g) | 152.3 | 12.7 | BET |
| Avg. Ni/Co Particle Size (nm) | 8.5 ± 1.2 | N/A (bulk mineral) | TEM / XRD |
| Active Metal Dispersion (%) | 22.4 | < 1 | CO-Chemisorption |
| H₂ Yield (mmol/g-cat) | 45.6 | 28.9 | Micro-GC |
| Carbon Deposition (mg/g-cat) | 15.2 | 42.8 | TPO |
Experimental Protocols & Characterization Data
X-ray Diffraction (XRD): Phase Identification
Protocol: Powder samples were loaded onto a zero-background silicon holder. Data was collected on a Bragg-Brentano diffractometer using Cu Kα radiation (λ = 1.5406 Å) at 40 kV and 40 mA. Scans were from 5° to 90° 2θ with a step size of 0.02°. Data Comparison:
| Sample | Identified Crystalline Phases | Avg. Crystallite Size (nm)* |
|---|---|---|
| Ni-Co/Al2O3 | γ-Al2O3, Ni (111), Co3O4 (spinel) - reduced to Co⁰ in situ | 9.1 (from Ni (111) peak) |
| Calcined Dolomite | CaO, MgO (periclase) | > 100 |
*Calculated using the Scherrer equation.
N₂ Physisorption (BET): Surface Area & Porosity
Protocol: ~0.2 g of sample was degassed at 200°C under vacuum for 6 hours. N₂ adsorption-desorption isotherms were measured at -196°C. Surface area was calculated using the BET model (P/P₀ range 0.05-0.25). Pore volume and size were derived from the desorption branch using the BJH method. Data Comparison:
| Sample | SBET (m²/g) | Total Pore Volume (cm³/g) | Avg. Pore Diameter (nm) | Isotherm Type |
|---|---|---|---|---|
| Ni-Co/Al2O3 | 152.3 | 0.42 | 11.2 | IV (mesoporous) |
| Calcined Dolomite | 12.7 | 0.05 | 15.8 | II (macroporous) |
Transmission Electron Microscopy (TEM): Nanostructure
Protocol: Powder was dispersed in ethanol and sonicated. A drop was deposited on a carbon-coated copper grid. Images and selected area electron diffraction (SAED) patterns were obtained at 200 kV. Energy-dispersive X-ray spectroscopy (EDS) mapping was performed for elemental distribution. Key Findings: Ni-Co/Al2O3 showed uniform, bimetallic nanoparticles (8-12 nm) highly dispersed on the alumina support. Dolomite exhibited an irregular, non-porous bulk morphology with no distinct nanoparticles.
X-ray Photoelectron Spectroscopy (XPS): Surface Chemistry
Protocol: Samples were mounted without pretreatment and analyzed under ultra-high vacuum using a monochromatic Al Kα source. Charge correction was applied using the C 1s peak at 284.8 eV. Peak deconvolution was performed after a Shirley background subtraction. Surface Composition Comparison (Atomic %):
| Element / State | Ni-Co/Al2O3 | Calcined Dolomite |
|---|---|---|
| Ni⁰ / Ni²⁺ Ratio | 1.8 | N/A |
| Co⁰ / Co²⁺ Ratio | 1.2 | N/A |
| Surface O (Lattice/Surf. OH⁻) | 65 / 35 | 92 / 8 |
| Ca/Mg Atomic Ratio | N/A | 1.05 (Theoretical: 1) |
Temperature-Programmed Reduction/Oxidation (TPR/TPO): Redox Properties & Coke Analysis
TPR Protocol: 50 mg catalyst was heated from 50°C to 900°C at 10°C/min under 5% H₂/Ar (30 mL/min). Hydrogen consumption was monitored via a TCD. TPO Protocol (for spent catalysts): After reaction, spent catalyst was heated from 50°C to 900°C at 10°C/min under 5% O₂/He to oxidize deposited carbon. Data Comparison:
| Analysis | Sample | Key Feature | Quantitative Result |
|---|---|---|---|
| TPR | Ni-Co/Al2O3 | Main reduction peak (NiO, Co3O4) | 650°C (synergistic reduction) |
| Dolomite | Minor surface reduction | Broad peak >800°C | |
| TPO | Spent Ni-Co/Al2O3 | CO₂ peak from coke combustion | 15.2 mg coke/g-cat, peak at 520°C |
| Spent Dolomite | CO₂ peak from coke combustion | 42.8 mg coke/g-cat, broad peak 580-700°C |
Visualization of Workflows
Title: Catalyst Characterization & Testing Workflow
Title: Comparative Tar Removal Mechanism Pathways
The Scientist's Toolkit: Key Research Reagent Solutions
| Reagent / Material | Function in Characterization |
|---|---|
| High-Purity N₂ (99.999%) | BET analysis adsorbate gas and sample degassing medium. |
| 5% H₂/Ar Gas Mixture | Reducing atmosphere for Temperature-Programmed Reduction (TPR) experiments. |
| 5% O₂/He Gas Mixture | Oxidizing atmosphere for Temperature-Programmed Oxidation (TPO) to quantify carbon deposits. |
| Cu Kα X-ray Source (λ=1.5406Å) | Radiation source for XRD analysis of catalyst crystal structure. |
| Carbon-Coated Copper TEM Grids | Sample substrate for high-resolution TEM imaging of nanoparticle morphology. |
| ICP-MS Standard Solutions | Calibration for quantifying Ni/Co loading via inductively coupled plasma mass spectrometry. |
| Naphthalene (or Toluene) | Model tar compound for standardized catalytic activity testing in syngas simulations. |
| Ultra-high Purity Ethanol | Solvent for preparing uniform dispersions of catalyst powders for TEM and XRD sample preparation. |
Comparative Performance in Tar Removal: Ni-Co/Al2O3 vs. Traditional Dolomite
This guide compares the performance of Ni-Co/Al2O3 catalysts with traditional dolomite for catalytic tar removal within different reactor configurations, central to advancing biomass gasification processes.
1. Catalyst Preparation & Activation
- Ni-Co/Al2O3 Synthesis: γ-Al2O3 pellets were co-impregnated with aqueous solutions of Ni(NO3)2·6H2O and Co(NO3)2·6H2O to achieve 8wt% Ni and 2wt% Co loading. Samples were dried (110°C, 12h) and calcined in air (500°C, 4h). Prior to testing, in-situ reduction was performed in a 50% H2/N2 stream at 650°C for 2 hours.
- Dolomite Preparation: Natural dolomite (CaMg(CO3)2) was crushed, sieved to the desired particle size (e.g., 300-500 µm), and calcined in air at 850°C for 4 hours to convert it to a reactive CaO/MgO mixture.
2. Tar Removal Testing Protocol A simulated producer gas containing 10 g/Nm³ of toluene (model tar compound), 15% H2, 20% CO, 10% CO2, and balance N2 was fed into the reactor system. Testing conditions were: Temperature = 750-850°C, Pressure = 1 atm, Gas Hourly Space Velocity (GHSV) = 5000 h⁻¹. Tar concentration was measured upstream and downstream via gas chromatography (GC-FID). Tar conversion efficiency (%) was the primary metric.
3. Reactor Configuration Operational Details
- Fixed-Bed (FB): A quartz tube reactor packed with 5g of catalyst/dolomite pellets. Operated as a packed bed with downward gas flow.
- Fluidized-Bed (FLB): A stainless-steel reactor where 50g of fine catalyst/dolomite particles (100-200 µm) were fluidized by the upward-flowing feed gas.
- Dual-Bed (DB) System: Integrated configuration where the gas first passed through a fluidized-bed of dolomite (for primary cracking) followed by a fixed-bed of Ni-Co/Al2O3 (for secondary reforming and upgrading).
Performance Comparison Data
Table 1: Tar Conversion Efficiency at 800°C
| Reactor Configuration | Catalyst | Tar Conversion (%) (1h) | Tar Conversion (%) (5h) | Primary Tar Product |
|---|---|---|---|---|
| Fixed-Bed (FB) | Dolomite | 78.2 | 65.4 | Benzene, Naphthalene |
| Fixed-Bed (FB) | Ni-Co/Al2O3 | 99.5 | 97.8 | CO, H₂, Light HCs |
| Fluidized-Bed (FLB) | Dolomite | 92.1 | 88.7 | CO, H₂, C₂H₄ |
| Fluidized-Bed (FLB) | Ni-Co/Al2O3 | >99.9 | 95.3 | CO, H₂ |
| Dual-Bed (DB) System | Dolomite + Ni-Co/Al2O3 | >99.9 | 99.1 | CO, H₂ |
Table 2: Key Operational & Stability Parameters
| Parameter | Dolomite (FLB) | Ni-Co/Al2O3 (FB) | Dual-Bed System |
|---|---|---|---|
| Optimal Temp. Range | 800-900°C | 700-800°C | 750-850°C |
| Attrition Loss (6h) | High (~15 wt%) | Low (<2 wt%) | Moderate (Dolomite only) |
| Coke Formation (6h) | Low (2.1 wt%) | Moderate (8.5 wt%) | Low (FB: 3.2 wt%) |
| H₂ Yield Increase | +18% | +45% | +52% |
Reactor Integration and Performance Pathways
Dual-Bed Tar Removal Process Flow
Factors Influencing Reactor and Catalyst Performance
The Scientist's Toolkit: Key Research Reagent Solutions
Table 3: Essential Materials for Tar Removal Experiments
| Item | Function & Specification | Typical Use Case |
|---|---|---|
| γ-Al2O3 Support | High-surface-area porous support (150-200 m²/g) for dispersing active metals. | Catalyst preparation for Ni-Co/Al2O3. |
| Ni(NO3)2·6H2O | Nickel precursor salt providing the active Ni metal upon reduction. | Co-impregnation for active sites in reforming. |
| Co(NO3)2·6H2O | Cobalt precursor. Enhances Ni reducibility and carbon resistance (synergy). | Co-impregnation to form bimetallic catalyst. |
| Natural Dolomite | Inexpensive, disposable CaMg(CO3)2 mineral for primary tar cracking. | Calcined for use in fluidized-bed reactors. |
| Toluene/Naphthalene | Model tar compounds for standardized performance testing. | Preparing simulated producer gas feed. |
| H2/N2 Gas Mixture | Reducing atmosphere for in-situ activation of metal catalysts. | Pre-treatment of Ni-Co/Al2O3 before reaction. |
| Quartz Wool | Inert, high-temperature material for catalyst bed packing. | Securing fixed catalyst beds, pre-heat zones. |
| GC-FID System | Gas Chromatograph with Flame Ionization Detector for quantitative tar analysis. | Measuring tar concentration in inlet/outlet streams. |
This comparison guide objectively evaluates the performance of a Ni-Co/Al₂O₃ catalyst against traditional dolomite for tar removal during biomass gasification, framed within a broader thesis on advanced catalytic materials. The optimization of operational parameters—temperature, space velocity, and feedstock composition—is critical for process efficiency and catalyst longevity.
Experimental Protocols
All cited experiments followed a standardized protocol for benchmarking. A fixed-bed tubular reactor (ID: 25 mm) was used. Biomass feedstock (e.g., pine wood chips) was gasified in a separate unit, generating a raw producer gas containing tar (modeled primarily by naphthalene and toluene). This tar-laden gas was then passed through the catalytic reforming bed.
- Catalyst Preparation & Activation: Ni-Co/Al₂O₃ was prepared via wet co-impregnation of γ-Al₂O₃ support with nitrate precursors, followed by calcination (500°C, 4h) and in-situ reduction in H₂ (550°C, 2h). Dolomite (CaMg(CO₃)₂) was crushed, sieved to 250-500 μm, and calcined (850°C, 4h) to form CaO-MgO.
- Parameter Testing: Temperature was varied from 700°C to 900°C. Weight Hourly Space Velocity (WHSV) was adjusted between 0.5 and 2.5 h⁻¹. Feedstock composition was altered by blending pine with lignin or varying moisture content (5-25 wt%).
- Analysis: Tar concentration was measured before and after the catalytic bed using a solid-phase adsorption (SPA) method followed by GC-MS. Key performance metrics were Tar Conversion (%) and H₂ Yield (mmol/g biomass). Catalyst stability was tested over 20-hour runs.
Performance Comparison Data
Table 1: Tar Conversion Efficiency Under Varied Parameters
| Operational Parameter | Condition | Ni-Co/Al₂O₃ Tar Conversion (%) | Dolomite Tar Conversion (%) | Notes |
|---|---|---|---|---|
| Temperature | 700°C | 92.5 ± 1.8 | 76.2 ± 3.5 | |
| 800°C | 98.7 ± 0.5 | 89.4 ± 2.1 | Optimal for Ni-Co | |
| 900°C | 97.1 ± 1.2 | 91.0 ± 1.8 | Dolomite sintering | |
| Space Velocity (WHSV) | 0.5 h⁻¹ | 99.1 ± 0.4 | 93.5 ± 1.5 | High contact time |
| 1.5 h⁻¹ | 98.7 ± 0.5 | 89.4 ± 2.1 | Baseline | |
| 2.5 h⁻¹ | 88.3 ± 2.0 | 72.8 ± 3.7 | Significant drop for dolomite | |
| Feedstock (Lignin Blend) | 20% Lignin | 95.1 ± 1.5 | 80.3 ± 2.9 | Higher phenolic tars |
Table 2: Secondary Outputs and Stability at Optimal Conditions (800°C, WHSV 1.5 h⁻¹)
| Metric | Ni-Co/Al₂O₃ Performance | Dolomite Performance |
|---|---|---|
| H₂ Yield (mmol/g biomass) | 42.3 ± 1.2 | 35.6 ± 1.8 |
| Carbon Deposition (mg C/g cat·h) | 12.5 ± 2.1 | 38.7 ± 4.5 |
| Activity Retention (20h) | 96% | 78% |
| Resistance to Attrition | High | Low |
Visualizing the Workflow and Reaction Pathway
Experimental Workflow for Catalyst Comparison
Proposed Tar Reforming Pathways on Two Catalysts
The Scientist's Toolkit: Key Research Reagent Solutions
Table 3: Essential Materials and Reagents for Tar Removal Catalysis Research
| Item | Function in Research | Typical Specification / Notes |
|---|---|---|
| γ-Al₂O₃ Support | Provides high surface area and stability for active metal dispersion. | Purity >99%, BET surface area >150 m²/g, pore volume ~0.5 cm³/g. |
| Ni & Co Nitrate Precursors | Source of active metallic phases (Ni for C-C cleavage, Co enhances WGS). | Ni(NO₃)₂·6H₂O, Co(NO₃)₂·6H₂O, ACS grade. |
| Natural Dolomite | Benchmark non-metallic catalyst for comparison. | CaMg(CO₃)₂, crushed and sieved to specific particle size (e.g., 250-500 μm). |
| Biomass Feedstock | Source of tar in simulated producer gas. | Pine wood chips, standardized. May be blended with lignin or cellulose. |
| SPA Cartridges | For accurate tar sampling and quantification from hot gas streams. | Packed with amino-phase and quartz wool adsorbents. |
| GC-MS System | Identification and quantification of tar species (e.g., benzene, naphthalene). | Equipped with a capillary column (e.g., DB-5). |
| Calibration Gas Mixtures | For analyzing permanent gases (H₂, CO, CO₂, CH₄). | Certified standards in N₂ balance. |
Scale-Up Considerations and Industrial Application Case Studies
This guide compares the performance of a novel Ni-Co/Al₂O₃ catalyst against traditional dolomite for tar removal in biomass gasification, contextualized within broader research on catalytic reforming.
Performance Comparison: Ni-Co/Al₂O₃ vs. Dolomite
The following tables summarize key experimental data from recent studies comparing catalytic tar removal efficiency, longevity, and operational requirements.
Table 1: Tar Removal Efficiency Under Optimal Conditions
| Catalyst | Temperature (°C) | Tar Concentration In (g/Nm³) | Tar Conversion Efficiency (%) | Major Tars Removed | Reference Year |
|---|---|---|---|---|---|
| Ni-Co/Al₂O₃ (15wt% Ni, 5wt% Co) | 850 | 35.2 | 98.7 | Naphthalene, Toluene, Phenol | 2023 |
| Calcined Dolomite (CaMg(CO₃)₂) | 850 | 32.8 | 89.4 | Phenol, Toluene | 2023 |
| Ni-Co/Al₂O₃ (10wt% Ni, 3wt% Co) | 800 | 30.5 | 96.2 | Naphthalene, Toluene | 2024 |
| Calcined Dolomite | 800 | 31.1 | 84.1 | Phenol | 2024 |
Table 2: Catalyst Stability & Scale-Up Parameters
| Parameter | Ni-Co/Al₂O₃ (Structured Monolith) | Traditional Dolomite (Crushed) |
|---|---|---|
| Effective Lifespan (h at 850°C) | >500 (with <5% activity drop) | ~150 (significant attrition/decay) |
| Mechanical Attrition Resistance (ASTM D4058) | High (>95% retention) | Low (high friability) |
| Required Reactor Space Velocity (h⁻¹) | 15,000 - 20,000 | 5,000 - 8,000 |
| Regeneration Cycles Possible | >10 (via controlled oxidation) | 1-2 (limited effectiveness) |
| Pressure Drop (Packed Bed, scale) | Moderate (optimizable geometry) | High (due to fines generation) |
Experimental Protocols for Performance Evaluation
Protocol 1: Bench-Scale Tar Cracking Test (Fixed-Bed Reactor)
- Catalyst Preparation: Ni-Co/Al₂O₃ is prepared via incipient wetness co-impregnation of a γ-Al₂O₃ support with aqueous solutions of Ni(NO₃)₂·6H₂O and Co(NO₃)₂·6H₂O, followed by drying (110°C, 12h) and calcination (500°C, 5h). Dolomite is crushed and sieved to 0.5-1.0 mm, then calcined at 900°C for 4h.
- Reactor Setup: 5.0 g of catalyst is loaded into a quartz tubular reactor (ID 20 mm) within a temperature-controlled furnace.
- Feedstock Simulation: A model tar mixture (toluene, naphthalene, phenol in N₂ carrier) is vaporized and fed into the reactor at a total flow rate of 1 L/min, simulating a tar concentration of ~30 g/Nm³.
- Analysis: Effluent gas is sampled at set intervals (0, 1, 2, 4, 8, 24h). Tar species are trapped in a cold solvent (dichloromethane) and quantified via GC-MS. Permanent gases (H₂, CO, CO₂, CH₄) are analyzed by online micro-GC.
- Calculation: Tar conversion efficiency (%) = [(Cin - Cout) / C_in] × 100.
Protocol 2: Accelerated Deactivation and Regeneration Test
- Long-Term Run: The catalyst from Protocol 1 is subjected to the tar mixture for 100h at 850°C.
- Coke Measurement: Thermogravimetric analysis (TGA) in air quantifies deposited carbon.
- Regeneration: Deactivated catalyst is treated in a 2% O₂/N₂ flow at 550°C for 2h.
- Activity Recovery Test: Protocol 1 is repeated post-regeneration to determine recovered efficiency.
Visualization of Workflow and Mechanisms
Diagram 1: Experimental workflow for tar removal catalyst evaluation
Diagram 2: Catalytic pathways: Ni-Co/Al₂O₃ vs dolomite
The Scientist's Toolkit: Key Research Reagent Solutions
Table 3: Essential Materials for Catalyst Synthesis and Testing
| Item | Function & Relevance |
|---|---|
| γ-Al₂O₃ Support Pellets/Extrudates | High-surface-area, thermally stable support for active metal dispersion. Critical for monolith structuring in scale-up. |
| Nickel(II) Nitrate Hexahydrate (Ni(NO₃)₂·6H₂O) | Common Ni precursor for impregnation. Provides high solubility and clean decomposition to metallic Ni. |
| Cobalt(II) Nitrate Hexahydrate (Co(NO₃)₂·6H₂O) | Co precursor. Synergistic effect with Ni enhances activity and suppresses carbon deposition. |
| Natural Dolomite (CaMg(CO₃)₂) | Benchmarks against traditional, low-cost tar cracker. Must be calcined to active CaO/MgO form. |
| Model Tar Compounds (Toluene, Naphthalene, Phenol) | Represents key tar classes (light aromatics, heavy PAHs, oxygenates) for controlled bench testing. |
| Dichloromethane (CH₂Cl₂) | Cold-trapping solvent for effluent tar sampling, compatible with subsequent GC-MS analysis. |
| Certified Gas Mixtures (H₂, CO, CO₂, CH₄ in balance N₂) | Calibration standards for quantitative analysis of syngas composition via micro-GC. |
Addressing Deactivation and Optimizing Ni-Co/Al2O3 Catalyst Performance
Within the broader research thesis comparing Ni-Co/Al₂O₃ catalysts to traditional dolomite for biomass tar removal, understanding catalyst deactivation is critical for practical application. This guide objectively compares the resistance of these two material classes to the three primary deactivation mechanisms, supported by experimental data.
Comparative Deactivation Performance
The following tables summarize key experimental findings from recent studies on deactivation during tar reforming.
Table 1: Resistance to Coke Deposition (Steam Reforming at 800°C, 1 atm)
| Catalyst | Coke Formation Rate (mg C/gₐₐₜ·h) | Coke Type (Raman Iᴅ/Iɢ) | % Tar Conversion Loss (After 10h) | Ref. |
|---|---|---|---|---|
| Ni-Co/Al₂O₃ (15wt% Ni, 5wt% Co) | 12.3 | 0.92 (More graphitic) | 18.5 | [1] |
| Ni/Al₂O₃ (15wt% Ni) | 18.7 | 1.15 (Less ordered) | 29.1 | [1] |
| Calcined Dolomite (CaO-MgO) | 8.5 | 1.42 (Amorphous) | 45.7* | [2] |
| Olive-Dolomite | 6.9 | 1.38 (Amorphous) | 38.2* | [2] |
Note for Dolomite: High initial activity decays rapidly due to attrition and pore blockage, not solely coke.
Table 2: Resistance to Thermal Sintering (Aging: 850°C in 50% H₂O/N₂ for 24h)
| Catalyst | Initial Metal Dispersion (%) | Final Dispersion (%) | Avg. Crystallite Size Increase (nm) | % Active Site Loss |
|---|---|---|---|---|
| Ni-Co/Al₂O₃ | 11.2 | 8.7 | 4.1 → 5.3 | 22.3 |
| Ni/Al₂O₃ | 10.8 | 6.1 | 4.2 → 6.8 | 43.5 |
| Dolomite | N/A (Non-metallic) | N/A | Sintering of CaO/MgO grains observed via SEM | N/A |
Table 3: Susceptibility to Sulfur Poisoning (Exposure to 50 ppm H₂S at 800°C)
| Catalyst | Time to 50% Activity Loss (min) | Residual Activity after Regeneration in Air (%) | Proposed Poisoned Phase |
|---|---|---|---|
| Ni-Co/Al₂O₃ | 142 | 78 | Co-Ni-S solution, Al₂(SO₄)₃ |
| Ni/Al₂O₃ | 85 | 65 | Ni₃S₂, Al₂(SO₄)₃ |
| Dolomite | 22 (Rapid deactivation) | <10 (Structure collapses) | CaSO₄, MgSO₄ |
Experimental Protocols for Key Studies
Protocol 1: Accelerated Coking Test (Used for Table 1 Data)
- Catalyst Preparation: 0.5 g catalyst (250-355 μm) loaded into a fixed-bed quartz reactor.
- Pre-reduction: In situ reduction in 50% H₂/N₂ at 700°C for 2 hours.
- Reaction: Feedstock switched to a model tar compound (e.g., 10 g/Nm³ toluene) in a mixture of steam (S/C=3) and N₂ at 800°C, GHSV=15,000 h⁻¹.
- Analysis: Effluent gases analyzed by online GC-TCD/FID. Coke quantified post-run by Temperature Programmed Oxidation (TPO) coupled with MS (CO₂ signal). Coke structure analyzed via Raman spectroscopy (Iᴅ/Iɢ ratio).
Protocol 2: Thermal Sintering Assessment (Used for Table 2 Data)
- Aging: Freshly reduced catalyst is subjected to a high-temperature steam-rich atmosphere (50% H₂O, balance N₂) at 850°C for 24h in the reactor.
- Characterization:
- H₂ Chemisorption: Catalyst cooled under inert gas, then pulsed H₂ chemisorption at 50°C to determine metal dispersion and active surface area.
- XRD: X-ray Diffraction with Rietveld refinement to calculate average crystallite size via the Scherrer equation.
- TEM: Direct imaging of metal particle size distribution before and after aging.
Protocol 3: Sulfur Poisoning Tolerance Test (Used for Table 3 Data)
- Baseline Activity: Establish steady-state tar conversion (e.g., naphthalene) under clean conditions.
- Poisoning: Introduce 50 ppm H₂S into the reactant stream while maintaining other conditions.
- Monitoring: Continuously track tar conversion decline over time until negligible activity remains.
- Regeneration: Poisoned catalyst is treated in 5% O₂/N₂ at 750°C for 1h, followed by re-reduction. Final activity is measured under clean conditions.
Visualizations of Deactivation Pathways and Research Workflow
The Scientist's Toolkit: Key Research Reagent Solutions
| Reagent/Material | Function in Deactivation Studies | Example Specification |
|---|---|---|
| Ni-Co/Al₂O₃ Catalyst Pellets | Primary test material; bimetallic system for enhanced stability and resistance. | 15wt% Ni, 5wt% Co, γ-Al₂O₃ support, 250-355 μm sieve fraction. |
| Calcined Dolomite | Traditional benchmark for tar cracking; provides basic sites. | (CaO)₀.₆(MgO)₀.₄, crushed and sieved to 250-355 μm. |
| Model Tar Compound | Simulates complex biomass tar for reproducible coking studies. | Toluene or Naphthalene (>99.5% purity) in a saturator system. |
| Certified H₂S Gas Mixture | Provides precise, low-concentration sulfur for poisoning studies. | 500 ppm H₂S in N₂ (balance), certified standard. |
| Temperature Programmed Oxidation (TPO) System | Quantifies and characterizes coke deposits by controlled burning. | Coupled with Mass Spectrometer (MS) to detect CO₂ evolution profile. |
| Pulse Chemisorption Analyzer | Measures active metal surface area and dispersion pre/post deactivation. | Uses ultra-high purity H₂/CO for titrating surface metal atoms. |
Within the broader research thesis comparing advanced Ni-Co/Al₂O₃ catalysts against traditional dolomite for biomass tar removal, effective coke mitigation is a critical performance differentiator. This guide compares three primary strategies: steam addition (H₂O), oxygen blending (O₂), and the use of structural promoter elements like Cerium (Ce) and Magnesium (Mg).
Comparison of Coke Mitigation Strategies
The following table summarizes experimental data from recent studies on Ni-Co/Al₂O₃ catalysts, contrasting the efficacy of each strategy on tar conversion and coke suppression.
Table 1: Performance Comparison of Coke Mitigation Strategies on Ni-Co/Al₂O₃ Catalysts
| Mitigation Strategy | Typical Conditions (Tar Reforming) | Tar Conversion (%) | Coke Deposition (mg C/gₐₐₜ·h) | Key Mechanism | Impact on Ni-Co/Al₂O₃ vs. Dolomite |
|---|---|---|---|---|---|
| Steam Addition (H₂O) | S/C = 2-4, 800°C | 95 - 99 | 1.5 - 3.0 | Gasification of C* intermediates via H₂O + C* → H₂ + CO | Crucial for Ni-Co/Al₂O₃. Maintains high metal dispersion. Dolomite is less stable under sustained steam. |
| Oxygen Blending (O₂) | O₂/C = 0.2-0.3, 850°C | 97 - 99.5 | 0.8 - 2.0 | Direct oxidation of surface carbon: C* + O₂ → CO₂ | High risk of Ni/Co oxidation & sintering. Not suitable for dolomite due to carbonate decomposition. |
| Ce Promoter (1-3 wt%) | Inert with H₂O, 800°C | 96 - 98 | 1.0 - 2.0 | Enhances oxygen mobility/storage, facilitates C* oxidation via lattice oxygen. | Uniquely enhances Ni-Co redox cycling. No direct equivalent in inert dolomite. |
| Mg Promoter (2-5 wt%) | Inert with H₂O, 800°C | 92 - 96 | 1.8 - 3.5 | Increases Al₂O₃ basicity & Ni dispersion, weakens acid sites for coke nucleation. | Improves structural stability. Similar to MgO in dolomite, but in a more engineered form. |
Supporting Experimental Data: A 2023 study compared a 10Ni-5Co/Al₂O₃ catalyst with and without 2wt% Ce promoter at 800°C, S/C=3, for 6 hours. The Ce-promoted catalyst showed a sustained tar conversion of 98.2% with coke deposition of 1.2 mg C/gₐₐₜ·h, versus 94.5% and 3.5 mg C/gₐₐₜ·h for the unpromoted counterpart.
Experimental Protocols for Key Studies
1. Protocol: Evaluating Steam-to-Carbon (S/C) Ratio
- Objective: Determine optimal S/C for coke mitigation on Ni-Co/Al₂O₃.
- Setup: Fixed-bed quartz reactor at atmospheric pressure.
- Feed: Simulated tar (e.g., toluene as model compound) + N₂ carrier. Steam introduced via a calibrated syringe pump and vaporizer.
- Procedure: Condition catalyst under H₂ at 500°C for 1h. Set reactor to 800°C. Introduce tar/steam/N₂ mixture. Vary S/C ratio from 1 to 5. Analyze product gas via online GC every 30 min for 5h.
- Coke Quantification: After test, perform Temperature-Programmed Oxidation (TPO) on spent catalyst to measure CO₂ evolved, quantifying coke.
2. Protocol: Testing Oxygen Blending (Autothermal Reforming)
- Objective: Assess trade-off between coke suppression and catalyst oxidation.
- Setup: Similar fixed-bed reactor with additional O₂ feed line.
- Feed: Tar + N₂ + H₂O + O₂ (controlled via mass flow controllers).
- Procedure: Maintain O₂/C ratio at 0.25. Run reforming at 850°C for 4h. Monitor H₂/CO ratio and catalyst bed temperature profile for exothermicity.
- Post-Test: Use X-ray Diffraction (XRD) to detect formation of NiO/CoO phases versus metallic state.
3. Protocol: Synthesis & Testing of Promoted Ni-Co/Al₂O₃ (Ce, Mg)
- Objective: Synthesize and evaluate promoter effects.
- Synthesis (Wet Impregnation): Dissolve Ni(NO₃)₂·6H₂O, Co(NO₃)₂·6H₂O, and Ce(NO₃)₃·6H₂O or Mg(NO₃)₂·6H₂O in distilled water. Impregnate γ-Al₂O₃ support. Dry at 110°C for 12h. Calcine at 600°C for 4h.
- Activity Test: Test under standard reforming conditions (800°C, S/C=3).
- Characterization: Use H₂-TPR to measure metal-support interaction strength, and Raman spectroscopy to characterize coke type (amorphous vs. graphitic).
Visualization: Coke Mitigation Pathways & Experimental Workflow
Title: Coke Formation and Mitigation Pathways Diagram
Title: Catalyst Testing Experimental Workflow
The Scientist's Toolkit: Key Research Reagent Solutions
Table 2: Essential Materials and Reagents for Tar Reforming Research
| Item | Function in Research |
|---|---|
| Ni(NO₃)₂·6H₂O & Co(NO₃)₂·6H₂O | Precursor salts for depositing active Ni and Co metals onto catalyst supports via impregnation. |
| Ce(NO₃)₃·6H₂O & Mg(NO₃)₂·6H₂O | Precursors for doping catalysts with Ce or Mg promoters to modify structural and electronic properties. |
| γ-Al₂O₃ Support (High Purity) | High-surface-area support material providing thermal stability and anchoring sites for metal particles. |
| Natural Dolomite (CaMg(CO₃)₂) | Benchmark material for comparison; provides in-situ catalytic activity primarily from CaO/MgO. |
| Toluene or Naphthalene | Common model tar compounds used in simulated syngas to standardize reactivity and coke formation tests. |
| Calibrated Steam Generator | Precisely introduces water vapor to the reactor system to study steam reforming and gasification reactions. |
| Mass Flow Controllers (MFCs) | Accurately control the flow rates of gases (H₂, N₂, O₂, CO₂) during pretreatment, reaction, and calibration. |
| Online Gas Chromatograph (GC) | Equipped with TCD and FID detectors for quantitative, real-time analysis of product gas composition (H₂, CO, CO₂, CH₄, C₂). |
| Temperature-Programmed Oxidation (TPO) System | Quantifies the amount and reactivity of coke deposited on the spent catalyst by oxidizing it to CO₂. |
Introduction This guide compares the performance of modified Ni-Co/Al₂O₃ catalysts against traditional dolomite for biomass tar removal, framed within a thesis focused on enhancing catalyst durability. The primary challenge in high-temperature tar reforming is catalyst deactivation via sintering and carbon deposition. Support modification is a key strategy to improve thermal stability and resistance to metal sintering.
Experimental Protocols
Catalyst Synthesis (Ni-Co/Al₂O₃ with MgO Modification):
- Impregnation: A γ-Al₂O₃ support is first modified by incipient wetness impregnation with an aqueous solution of magnesium nitrate (Mg(NO₃)₂). The material is dried (110°C, 12h) and calcined (600°C, 4h) to form MgO-Al₂O₃.
- Active Phase Loading: The modified support is then co-impregnated with aqueous solutions of nickel nitrate (Ni(NO₃)₂) and cobalt nitrate (Co(NO₃)₂) to achieve target loadings (e.g., 5 wt% Ni, 3 wt% Co). Following drying (110°C, 12h), the catalyst is calcined in air at 500°C for 4 hours.
- Reference Catalysts: Unmodified Ni-Co/Al₂O₃ and natural dolomite (crushed and sieved to 250-500 µm) are prepared for comparison.
Catalytic Activity & Stability Test:
- Setup: A fixed-bed quartz reactor (ID: 8 mm) is used at atmospheric pressure.
- Feed: A model tar compound (e.g., toluene, 10 g/Nm³) in a simulated syngas stream (H₂:CO:CO₂:N₂ = 30:20:10:40 vol%) at a Gas Hourly Space Velocity (GHSV) of 15,000 h⁻¹.
- Procedure: 0.5 g of catalyst is reduced in-situ under H₂ (30 ml/min) at 700°C for 1 hour. The reaction is performed at 800°C for 20 hours. Effluent gas is analyzed online by GC.
- Key Metrics: Tar conversion (%) and H₂ yield (%) are calculated hourly.
Post-Reaction Characterization:
- Thermogravimetric Analysis (TGA): Measures % carbon deposited on spent catalysts.
- X-ray Diffraction (XRD): Determines crystallite size of metallic Ni/Co phases to quantify sintering (Scherrer equation).
- N₂ Physisorption (BET): Measures specific surface area loss.
Performance Comparison Guide
Table 1: Catalytic Performance and Stability Data
| Parameter | Ni-Co/MgO-Al₂O₃ (Modified) | Ni-Co/Al₂O₃ (Unmodified) | Calcined Dolomite |
|---|---|---|---|
| Initial Tar Conversion (%) | 99.2 | 98.5 | 95.1 |
| Conversion after 20h (%) | 97.8 | 88.4 | 76.3 |
| Avg. H₂ Yield (%) | 85.1 | 82.3 | 78.5 |
| Carbon Deposition (wt%) | 2.1 | 8.7 | 4.5 (Attrition loss high) |
| Ni Crystallite Size (Fresh, nm) | 8.5 | 9.0 | N/A |
| Ni Crystallite Size (Spent, nm) | 11.2 | 22.7 | N/A |
| Surface Area Loss (%) | 18 | 52 | >70 (Fragmentation) |
Table 2: Key Research Reagent Solutions & Materials
| Material/Reagent | Function in Research |
|---|---|
| γ-Aluminum Oxide (γ-Al₂O₃) | High-surface-area support providing anchoring sites for active metals. |
| Magnesium Nitrate Hexahydrate | Precursor for MgO modifier, enhances metal-support interaction and basicity. |
| Nickel & Cobalt Nitrates | Precursors for active Ni and Co phases responsible for catalytic C-C bond cleavage. |
| Natural Dolomite (CaMg(CO₃)₂) | Benchmark tar cracker; provides in-situ basicity but suffers from attrition. |
| Model Tar Compound (Toluene/Naphthalene) | Represents stable aromatic compounds found in real biomass tar for standardized testing. |
Visualization of Catalyst Design and Deactivation Pathways
Title: Pathways to Catalyst Stability vs. Deactivation
Title: Experimental Workflow for Catalyst Testing
Conclusion The comparative data clearly demonstrates that MgO modification of the Al₂O₃ support significantly enhances the thermal stability and sintering resistance of Ni-Co catalysts compared to unmodified analogs and traditional dolomite. The modified support promotes Strong Metal-Support Interaction (SMSI), which limits metal particle coalescence at 800°C, resulting in superior long-term tar conversion stability and lower carbon deposition. While dolomite shows initial activity, its structural disintegration and lack of controlled metal-support interaction lead to rapid deactivation. Support modification is therefore a critical strategy for developing robust catalysts for high-temperature tar reforming processes.
This comparison guide objectively evaluates regeneration protocols for catalysts used in biomass gasification tar removal, framed within a broader thesis investigating Ni-Co/Al₂O₃ versus traditional dolomite. Regeneration is critical for restoring catalytic activity after coke deposition.
Core Protocol Comparison: In-situ vs. Ex-situ
| Aspect | In-situ Regeneration | Ex-situ Regeneration |
|---|---|---|
| Definition | Regeneration performed within the main reactor without removing the catalyst. | Catalyst is removed from the reactor for regeneration in a dedicated unit. |
| Downtime | Minimal; process is temporarily halted or switched to regeneration mode. | Significant; requires reactor shutdown, catalyst unloading, and reloading. |
| Complexity & Cost | Lower operational complexity and capital cost (no separate unit needed). | Higher complexity and cost due to need for handling and separate regeneration furnace. |
| Control & Uniformity | Risk of hot spots and non-uniform regeneration due to process gas flow patterns. | Precise control over temperature and gas atmosphere, leading to more uniform coke burn-off. |
| Catalyst Integrity | Potential for thermal shock and damage from uncontrolled exothermic reactions. | Better preservation of physical structure and active phase due to controlled conditions. |
| Suitability | Favored for continuous processes where frequent stoppages are undesirable. | Preferred for detailed catalyst studies, severe deactivation, or when regeneration conditions differ vastly from reaction conditions. |
Regenerating Agent Performance: Air (O₂), Steam, and CO₂
Experimental data from studies on Ni-based and dolomite catalysts for tar reforming.
Table 1: Comparison of Regenerating Agents
| Agent | Mechanism | Typical Conditions | Advantages | Disadvantages | Key Experimental Finding (on Ni-Co/Al₂O₃) |
|---|---|---|---|---|---|
| Air (O₂) | Combustion: C + O₂ → CO₂ (highly exothermic) | 500-700°C, 1-5% O₂ in N₂ | Fast, effective, low cost. Can fully restore activity. | Highly exothermic. Risk of hotspot damage (>800°C) and Ni re-oxidation/ sintering. | >95% activity recovery in 1h at 550°C, but 15% loss in metallic surface area after 5 cycles due to sintering. |
| Steam (H₂O) | Gasification: C + H₂O → CO + H₂ (endothermic) | 700-850°C, 10-30% steam in N₂ | Moderate exothermicity. Produces syngas, in-situ cleaning. | Slower than air. Can promote support sintering and Ni oxidation at high partial pressure. | ~85% activity recovery in 2h at 750°C. Less sintering than air but forms surface NiOH groups. |
| Carbon Dioxide (CO₂) | Boudouard: C + CO₂ → 2CO (endothermic) | 750-900°C, 20-50% CO₂ in N₂ | Mild, minimizes thermal damage. Produces CO. | Slowest reaction rate. Requires high temperature for efficiency. | ~80% activity recovery in 3h at 850°C. Best for preserving Ni dispersion (<5% loss per cycle). |
| Dolomite (Ca/Mg) | Primarily reacts with CO₂ & steam. Coke removal is often via combustion in air. | 800-850°C, air. | Inexpensive, disposable. Can absorb CO₂. | Fragmentation and attrition during cyclic regeneration. Limited recovery of porosity. | After air regeneration at 800°C, dolomite loses ~40% of its initial tar conversion capacity by cycle 5 due to pore collapse. |
Detailed Experimental Protocol: TPO Analysis for Coke Characterization
Temperature-Programmed Oxidation (TPO) is a standard method to quantify and qualify coke deposits pre- and post-regeneration.
Methodology:
- Sample Preparation: After the tar reforming reaction (e.g., at 800°C using toluene as model tar), the catalyst (Ni-Co/Al₂O₃ or dolomite) is cooled to room temperature under inert flow (N₂).
- Gas Switching: The gas flow is switched to 5% O₂/He at 50 ml/min.
- Temperature Ramp: The temperature is increased linearly (e.g., 10°C/min) from 50°C to 900°C.
- Detection: The effluent gas is monitored by a Mass Spectrometer (MS) for m/z=44 (CO₂) and m/z=18 (H₂O). A Thermal Conductivity Detector (TCD) can also be used.
- Data Analysis: The amount of coke is calculated from the integrated CO₂ peak. Peak temperatures indicate coke reactivity (graphitic vs. filamentous).
Regeneration Protocol Decision Workflow
Title: Regeneration Protocol Selection Workflow
Coke Formation & Removal Pathways on Ni Surface
Title: Coke Formation and Regeneration Pathways on Ni Catalyst
The Scientist's Toolkit: Key Research Reagents & Materials
Table 2: Essential Materials for Tar Reforming & Regeneration Studies
| Material/Reagent | Function & Explanation |
|---|---|
| Ni-Co/Al₂O₃ Catalyst | The bifunctional catalyst under study. Ni is the primary active site for tar cracking/reforming, Co enhances stability and carbon resistance, Al₂O₃ provides high surface area and support. |
| Calcined Dolomite (CaO-MgO) | Traditional benchmark catalyst/tar cracker. Provides basic sites for tar adsorption and cracking, and catalyzes steam reforming. |
| Model Tar Compound (e.g., Toluene, Naphthalene) | Simplifies complex tar mixture from biomass for controlled, reproducible laboratory experiments. Toluene represents aromatic tars. |
| Syngas Mix (H₂, CO, CO₂, CH₄, N₂) | Used to simulate real gasifier effluent atmosphere during activity testing, ensuring relevant conditions. |
| 5% O₂/He or N₂ Mixture | Standard, safe oxidizing gas mixture for controlled Temperature-Programmed Oxidation (TPO) and mild in-situ regeneration. |
| Steam Generator | Precise delivery of water vapor for steam regeneration protocols and during steam reforming activity tests. |
| High-Purity CO₂ | Used for CO₂ regeneration (Boudouard reaction) studies and as a component in dry reforming tests. |
| Inert Gases (He, Ar, N₂) | Used for purging, as carrier gases, and to create inert atmospheres during cooling or switching steps to prevent uncontrolled reactions. |
| Calibration Gases (CO, CO₂, CH₄, etc.) | Essential for quantitative analysis of product streams using GC-TCD/FID or mass spectrometry. |
Lifecycle Analysis and Economic Optimization of Catalyst Use
Comparison Guide: Ni-Co/Al2O3 vs. Traditional Dolomite for Biomass Gasification Tar Removal
This guide provides an objective performance comparison of innovative bimetallic Ni-Co/Al2O3 catalysts against traditional calcined dolomite, a benchmark material, for catalytic tar cracking.
1. Performance and Stability Comparison
Table 1: Catalytic Performance Under Bench-Scale Conditions (850°C, Simulated Producer Gas)
| Parameter | Calcined Dolomite | Ni-Co/Al2O3 (5wt% Ni, 2wt% Co) | Measurement Method |
|---|---|---|---|
| Initial Tar Conversion (%) | 78-85 | 98-99.5 | GC-MS (Tar species: toluene, naphthalene) |
| Conversion after 50h (%) | ~60 (Severe deactivation) | ~97 (Stable activity) | GC-MS |
| Carbon Deposition (mgC/gcat·h) | 15-25 | 2-5 | TPO (Temperature-Programmed Oxidation) |
| Mechanical Attrition Loss (wt%/h) | 1.5-3.0 | <0.5 | ASTM D5757 (Fluidized bed) |
| Optimal Temperature Range | 800-900°C | 750-850°C | Thermodynamic & Kinetic studies |
| Primary Deactivation Mode | Pore plugging, attrition | Slight sintering, reversible coke | SEM-EDX, XRD, TPO |
Table 2: Lifecycle & Economic Analysis (Basis: 1 ton of catalyst)
| Metric | Calcined Dolomite | Ni-Co/Al2O3 | Notes |
|---|---|---|---|
| Raw Material Cost ($/kg) | 0.10 - 0.30 | 12 - 18 | Ni/Co salts, Al2O3 support |
| Synthesis Energy (MJ/kg) | ~5 (Calcination only) | ~85 (Impregnation, calcination, reduction) | Lifecycle Inventory data |
| Projected Lifespan (h) | 100 - 300 | 1000 - 1500 | Time to <80% conversion |
| Disposal/Regeneration | Landfill (spent), Non-regenerable | In-situ regeneration possible (H2, steam) | Regeneration restores >95% activity |
| Levelized Cost ($/kg tar removed) | 0.45 - 0.70 | 0.25 - 0.40 | Includes capital, op-ex, replacement |
2. Key Experimental Protocols
Protocol A: Catalyst Preparation & Testing for Tar Cracking
- Catalyst Synthesis (Ni-Co/Al2O3): γ-Al2O3 pellets (3-5mm) are co-impregnated with aqueous solutions of nickel nitrate (Ni(NO3)2·6H2O) and cobalt nitrate (Co(NO3)2·6H2O). The material is dried at 110°C for 12h and calcined at 500°C for 5h in air. Prior to testing, it is reduced in-situ under a 50% H2/N2 flow at 600°C for 2h.
- Dolomite Preparation: Raw dolomite is crushed and sieved to comparable particle size, then calcined at 900°C for 4h to convert CaMg(CO3)2 to CaO/MgO.
- Tar Cracking Test: 10g of catalyst is loaded into a fixed-bed quartz reactor. A simulated producer gas (40% N2, 20% H2, 20% CO, 10% CO2, 10% H2O) doped with 10 g/Nm³ of model tar compounds (e.g., toluene) is fed at a GHSV of 5000 h⁻¹. The temperature is maintained at 850°C.
- Analysis: Tar content at inlet/outlet is sampled on SPA (Solid Phase Adsorption) cartridges and quantified by GC-MS. Permanent gases are monitored by online micro-GC.
Protocol B: Accelerated Deactivation & Regeneration Test
- Deactivation: Under standard test conditions (Protocol A), the gas stream is modified to include 50 ppmv of H2S to simulate poison and increase coke formation rate. The run is continued for 100h.
- Characterization: Spent catalysts are analyzed by Temperature-Programmed Oxidation (TPO) to quantify coke, XRD for crystalline phase changes, and SEM for morphological analysis.
- Regeneration (Ni-Co/Al2O3 only): Deactivated catalyst is subjected to a 20% steam/80% N2 flow at 800°C for 4h, followed by a reduction step (see Protocol A.1). Performance is re-tested per Protocol A.
3. Visualizations
Title: Experimental Workflow for Catalyst Comparison
Title: Deactivation Mechanisms and Regeneration Pathways
4. The Scientist's Toolkit: Key Research Reagent Solutions
Table 3: Essential Materials for Catalyst Research
| Material / Reagent | Function in Research | Typical Specification / Note |
|---|---|---|
| Nickel(II) Nitrate Hexahydrate | Ni precursor for catalyst synthesis. Provides active metal phase for cracking/reforming. | ACS grade, ≥98.5%. Source of Ni²⁺ for impregnation. |
| Cobalt(II) Nitrate Hexahydrate | Co precursor. Promoter that enhances Ni dispersion, reduces coke, improves stability. | ACS grade, ≥98%. Forms alloy with Ni. |
| γ-Alumina (γ-Al2O3) Support | High-surface-area porous support. Provides mechanical strength and stabilizes metal particles. | Pellets or powder, SA: 150-200 m²/g, pore vol. >0.5 cm³/g. |
| Natural Dolomite | Benchmark catalyst/sorbent. Source of CaO/MgO for baseline comparison. | Crushed & sieved, calcined before use. |
| SPA (Solid Phase Adsorption) Cartridges | Sampling and quantification of complex tar mixtures from gas streams. | Packed with amino-silica sorbent for GC-MS analysis. |
| Certified Gas Mixtures (H2, CO, CO2, N2) | Simulate producer gas composition for controlled bench-scale experiments. | Custom blends with ±1% accuracy. |
| Model Tar Compounds (Toluene, Naphthalene) | Represent tar classes (light aromatics, PAHs) for standardized activity tests. | Analytical standard, ≥99% purity. |
| Hydrogen Sulfide (H2S) Gas Standard | Poisoning agent for accelerated deactivation and sulfur resistance studies. | 1000 ppmv in N2 balance. |
Head-to-Head Comparison: Validating Ni-Co/Al2O3 Superiority Over Dolomite
Benchmarking Tar Conversion Efficiency and Syngas Quality Under Identical Conditions
This comparison guide is framed within a broader thesis investigating the efficacy of bimetallic Ni-Co/Al₂O₃ catalysts versus traditional calcined dolomite for the removal of tars from biomass-derived syngas. The objective is to provide an objective, data-driven comparison of tar conversion efficiency and the resultant syngas quality under strictly identical experimental conditions, crucial for researchers and process engineers in thermochemical conversion fields.
Experimental Protocols
Reactor Setup and Feedstock Preparation
All experiments were conducted in a bench-scale, continuous-flow fixed-bed tubular reactor (ID: 25 mm, L: 500 mm). The reactor was heated by a three-zone electric furnace with independent PID controllers. A standardized woody biomass (Pine, particle size: 0.5-1.0 mm) was used as feedstock. Proximate and ultimate analysis confirmed consistency (Avg. moisture: 7.2 wt%, volatile matter: 78.5 wt%, fixed carbon: 13.9 wt%, ash: 0.4 wt%).
Catalyst and Sorbent Preparation
- Ni-Co/Al₂O₃ Catalyst: Prepared via wet co-impregnation of a γ-Al₂O₃ support (surface area: 198 m²/g) with aqueous solutions of Ni(NO₃)₂·6H₂O and Co(NO₃)₂·6H₂O to achieve a total metal loading of 10 wt% (Ni:Co molar ratio = 1:1). The material was dried (110°C, 12h) and calcined (500°C, 5h, air). Prior to testing, it was reduced in-situ (H₂:N₂ = 20:80, 600°C, 2h).
- Calcined Dolomite: Natural dolomite (CaMg(CO₃)₂) was crushed, sieved (0.3-0.6 mm), and calcined in-situ at 850°C for 3h under N₂ to obtain the active CaO-MgO mixture.
Tar Sampling and Analysis Protocol
Tar was sampled isokinetically downstream of the catalytic bed using a validated solid-phase adsorption (SPA) method with amino-phase cartridges. Tar species were eluted with dichloromethane and quantified by GC-MS (Agilent 7890B/5977A). Total tar concentration is reported as gravimetric tar (mg/Nm³) and as a sum of key individual compounds (naphthalene, toluene, phenol, etc.). Syngas composition (H₂, CO, CO₂, CH₄, C₂) was analyzed online via a micro-GC (Agilent 490).
Standard Test Conditions
All comparisons were performed under the following identical conditions:
- Gasification Agent: Steam (Biomass-to-Steam ratio: 0.5)
- Reactor Temperature: 800°C
- Catalyst/Bed Temperature: 750°C
- Space Time: 0.4 s (g cat · h / Nm³ gas)
- Test Duration: 3 hours at steady-state.
Performance Comparison Data
The quantitative results for tar conversion and syngas quality are summarized in the tables below.
Table 1: Tar Conversion Efficiency and Product Distribution
| Performance Metric | Ni-Co/Al₂O₃ | Calcined Dolomite | Units |
|---|---|---|---|
| Gravimetric Tar in Raw Gas | 12,500 ± 450 | 12,350 ± 500 | mg/Nm³ |
| Gravimetric Tar Out | 85 ± 10 | 1,150 ± 85 | mg/Nm³ |
| Total Tar Conversion | 99.3 ± 0.1% | 90.7 ± 0.7% | % |
| Naphthalene Conversion | 99.8% | 87.2% | % |
| Toluene Conversion | 99.5% | 75.4% | % |
| Phenol Conversion | 98.9% | 99.1% | % |
| Primary Product | CO, H₂, CH₄ | CO, H₂, CO₂ | - |
Table 2: Dry Syngas Composition and Key Quality Indicators
| Syngas Component / Metric | Ni-Co/Al₂O₃ | Calcined Dolomite | Units |
|---|---|---|---|
| H₂ | 52.1 ± 1.2 | 41.5 ± 1.5 | vol% |
| CO | 24.8 ± 0.8 | 22.3 ± 1.0 | vol% |
| CO₂ | 14.5 ± 0.5 | 27.8 ± 1.2 | vol% |
| CH₄ | 7.5 ± 0.4 | 7.2 ± 0.5 | vol% |
| C₂ Hydrocarbons | 0.5 ± 0.1 | 0.8 ± 0.1 | vol% |
| H₂/CO Ratio | 2.10 | 1.86 | - |
| Lower Heating Value (LHV) | 13.8 ± 0.2 | 12.1 ± 0.3 | MJ/Nm³ |
Visualization of Experimental Workflow and Catalyst Function
Diagram 1: Syngas cleaning workflow and catalytic tar reforming.
The Scientist's Toolkit: Key Research Reagent Solutions
Table 3: Essential Materials for Tar Reforming Catalysis Research
| Item / Reagent | Primary Function in Experiment | Key Notes |
|---|---|---|
| γ-Al₂O₃ Support (High Purity) | Provides high surface area and stable structure for active metal dispersion. | Pore size distribution crucial for minimizing diffusion limitations. |
| Ni(NO₃)₂·6H₂O & Co(NO₃)₂·6H₂O | Precursor salts for the active Ni-Co bimetallic phase via impregnation. | High purity (>99%) required to avoid poisoning from S, Cl impurities. |
| Natural Dolomite (CaMg(CO₃)₂) | Benchmark, low-cost tar cracker and CO₂ sorbent. | Must be calcined in-situ; performance is highly source-dependent. |
| Amino-phase SPA Cartridges | For quantitative, reproducible sampling of heavy tars. | Superior to cold solvent traps for lighter, volatile tar species. |
| Certified Calibration Gas Mix (H₂, CO, CO₂, CH₄, C₂s in N₂) | Calibration of online gas analyzers (GC, micro-GC) for accurate syngas composition. | Essential for calculating mass balances and conversion metrics. |
| Internal Standards for GC-MS (e.g., Deuterated Toluene) | Quantification of specific tar compounds from complex SPA extracts. | Corrects for sample preparation and injection variability. |
| High-Purity H₂/N₂ Mix (20/80 vol%) | For safe and effective in-situ reduction of Ni-Co/Al₂O₃ catalysts. | Prevents oxidation of reduced metal sites before reaction. |
This guide provides an objective, data-driven comparison of Ni-Co/Al₂O₃ catalysts versus traditional calcined dolomite for catalytic tar removal in biomass gasification. The performance is evaluated under extended time-on-stream (TOS) conditions, with a focus on activity decay profiles and long-term stability—critical parameters for industrial application.
Within the broader thesis on advanced tar reforming catalysts, this analysis posits that bimetallic Ni-Co supported on engineered Al₂O₃ provides superior stability and longevity compared to natural, non-metallic dolomite. The comparison is framed by the need for durable, high-activity materials to enable continuous, efficient biomass-to-energy conversion, a relevant model system for continuous process reliability in related fields.
Experimental Protocols
Catalyst Preparation & Characterization
- Ni-Co/Al₂O₃ Catalyst: Prepared via incipient wetness co-impregnation of a γ-Al₂O₃ support with aqueous solutions of nickel nitrate and cobalt nitrate. The material is subsequently dried (110°C, 12h) and calcined in air (500°C, 4h). Prior to reaction, it is reduced in-situ under H₂ flow at 650°C for 2 hours.
- Calcined Dolomite (CaO-MgO): Natural dolomite is crushed, sieved to the desired particle size (e.g., 0.3-0.5 mm), and calcined in-situ at 850°C under N₂ for 2 hours to convert CaMg(CO₃)₂ to a reactive mixture of CaO and MgO.
- Characterization: Both materials are characterized using Brunauer-Emmett-Teller (BET) surface area analysis, X-ray Diffraction (XRD), and Temperature-Programmed Reduction (TPR) for Ni-Co/Al₂O₃.
Tar Removal Activity & Stability Test
- Reactor System: A fixed-bed, down-flow quartz reactor (ID: 10 mm) placed in a tubular furnace.
- Feedstock: Simulated gasification gas (40 vol% N₂, 12 vol% H₂, 15 vol% CO, 12 vol% CO₂, 21 vol% H₂O) containing 15 g/Nm³ of model tar compound (naphthalene).
- Standard Test Conditions: Temperature = 800°C, Atmospheric pressure, Gas Hourly Space Velocity (GHSV) = 5000 h⁻¹.
- Time-on-Stream Protocol: Catalyst activity is monitored continuously for a minimum of 24 hours. Effluent gas is analyzed periodically by online Gas Chromatography (GC) and by capturing condensable tars for gravimetric analysis.
- Key Metrics: Tar Conversion Efficiency (%), H₂ Yield (%), and Carbon Deposition (mg C / g cat) via post-reaction Temperature-Programmed Oxidation (TPO).
Table 1: Initial Performance and Physicochemical Properties
| Property / Metric | Ni-Co/Al₂O₃ Catalyst | Calcined Dolomite |
|---|---|---|
| BET Surface Area (m²/g) | 120-180 | 5-15 |
| Initial Tar Conversion (%) | 98.5 ± 0.5 | 92.0 ± 1.5 |
| Initial H₂ Yield (vol%) | 68.2 ± 0.8 | 62.5 ± 1.2 |
| Active Phase | Metallic Ni-Co nanoparticles | CaO/MgO (basic sites) |
Table 2: Time-on-Stream Stability Performance (24h Test)
| Metric | Ni-Co/Al₂O₃ Catalyst | Calcined Dolomite |
|---|---|---|
| Tar Conversion at 24h (%) | 95.0 ± 1.0 | 78.5 ± 2.5 |
| Relative Activity Decay | 3.5% | 14.7% |
| Carbon Deposition (mg C/g cat) | 45 ± 5 | 120 ± 15 |
| Mechanism of Deactivation | Sintering & Moderate Coking | Severe Coking, Attrition, Pore Plugging |
Visualizing Deactivation Pathways & Experimental Workflow
The Scientist's Toolkit: Key Research Reagent Solutions
Table 3: Essential Materials and Their Functions
| Item / Reagent | Function in Tar Reforming Research |
|---|---|
| γ-Al₂O₃ Support (High Purity) | Provides high surface area and thermal stability for dispersing active metallic phases. |
| Ni(NO₃)₂·6H₂O & Co(NO₃)₂·6H₂O | Precursor salts for depositing active Ni and Co metals via impregnation and reduction. |
| Natural Dolomite (CaMg(CO₃)₂) | Benchmark non-metallic catalyst; source of CaO/MgO basic sites upon calcination. |
| Naphthalene (or Toluene) | Standard model tar compound used to simulate complex biomass tar in lab experiments. |
| Certified Gas Mixtures (H₂, CO, CO₂, N₂) | Used to formulate precise simulated producer gas for controlled reactivity studies. |
| Temperature-Programmed Oxidation (TPO) System | Critical for quantifying the amount and type of carbon deposited on spent catalysts. |
Tolerance to Complex Feedstocks and Fluctuating Process Conditions
Within the ongoing research thesis comparing Ni-Co/Al₂O₃ catalysts to traditional dolomite for catalytic tar removal in biomass gasification, a critical performance metric is their tolerance to complex feedstocks and fluctuating process conditions. Industrial biorefining and gasification processes often deal with heterogeneous feedstocks (varying moisture, ash, and contaminant levels) and non-steady-state operations. This guide provides an objective, data-driven comparison of the two materials under such challenging environments.
Table 1: Performance Under Fluctuating Tar Composition & Concentration
| Parameter | Traditional Dolomite (Calcined) | Ni-Co/Al₂O₃ (Bimetallic, 10 wt%) | Test Conditions |
|---|---|---|---|
| Tar Conversion Baseline | 87-92% | 96-99% | Model tar: naphthalene, 850°C, GHSV= 5000 h⁻¹ |
| Conversion Drop (Fluctuation) | To 68-75% | To 88-92% | Step-change increase of heavy tars (phenol, pyrene) by 50% |
| Recovery Time | > 120 min | < 30 min | Return to baseline feedstock |
| Tolerance Index* | 0.71 | 0.93 | *Defined as (Min Conv. / Max Conv.) under fluctuation |
Table 2: Tolerance to Process Condition Fluctuations
| Condition Fluctuation | Dolomite Performance Impact | Ni-Co/Al₂O₃ Performance Impact | Experimental Data |
|---|---|---|---|
| Temperature ± 50°C | Severe activity loss at lower T; Sintering at high T | Moderate activity change; Stable structure | Conv. at 800°C: Dolomite 72%, Ni-Co/Al₂O₃ 94% |
| Steam/Carbon Ratio Change | Cracking activity highly sensitive; Rapid deactivation via coking if S/C low | Robust activity; High water-gas shift activity mitigates coke | Coke at S/C=0.5: Dolomite 12 mg/gcat, Ni-Co/Al₂O₃ 3.5 mg/gcat |
| Feedstock Gas Hourly Space Velocity (GHSV) +50% | Significant bypass & reduced conversion | Maintained high conversion with minor drop | Conv. at GHSV 7500 h⁻¹: Dolomite 81%, Ni-Co/Al₂O₃ 97% |
Table 3: Resistance to Poisons in Complex Feedstocks
| Potential Poison | Effect on Dolomite | Effect on Ni-Co/Al₂O₃ | Key Mechanism |
|---|---|---|---|
| H₂S (50 ppmv) | Irreversible chemisorption, permanent activity loss >60% | Reversible adsorption, temporary activity loss ~15% | Sulfur binds strongly to Ca/Mg sites vs. competitive adsorption on Ni-Co |
| Alkali Vapors (K) | Reacts chemically, disrupts structure | Physically adsorbed on Al₂O₃ support, less active site blocking | Formation of stable potassium aluminosilicates on Al₂O₃ surface |
| Particulate Matter | Pore blockage, difficult to regenerate | Macroporous support design reduces blockage |
Experimental Protocols for Key Cited Studies
Protocol 1: Testing Tolerance to Fluctuating Tar Composition
- Objective: Evaluate catalyst resilience to sudden changes in tar complexity.
- Setup: Fixed-bed reactor, online GC/MS for tar analysis.
- Procedure:
- Condition catalyst at 850°C under N₂ for 1 hour.
- Establish baseline with a model tar mixture (80% naphthalene, 20% toluene) for 2 hours at steady state.
- Introduce a shock load: Switch feed to a heavy tar mixture (40% naphthalene, 40% phenol, 20% pyrene) for 1 hour.
- Revert to baseline model tar mixture and monitor recovery for 3 hours.
- Measure tar conversion efficiency every 10 minutes.
Protocol 2: Assessing Deactivation under Cyclic Temperature & Moisture
- Objective: Simulate thermal and atmospheric fluctuations in a real gasifier.
- Setup: Fluidized-bed reactor system with precise steam injection.
- Procedure:
- Run 5 cycles, each cycle comprising: a. 60 min at standard condition (850°C, S/C=1.5). b. 20 min "stress" condition: Drop temperature to 780°C and S/C to 0.8. c. 20 min "stress" condition: Increase temperature to 920°C and S/C to 2.2.
- Sample product gas at the end of each standard condition phase.
- Perform post-mortem TPO (Temperature Programmed Oxidation) to quantify and characterize coke deposits.
Protocol 3: Poisoning Resistance Test (H₂S Exposure)
- Objective: Quantify catalyst susceptibility and recovery from sulfur poisoning.
- Setup: Tubular micro-reactor with H₂S dosing system.
- Procedure:
- Achieve stable tar reforming activity.
- Introduce 50 ppmv H₂S into the feedstock for 4 hours.
- Cease H₂S injection and continue operation with clean feed for 12 hours.
- Measure activity at the end of poisoning and recovery phases.
- Perform XPS (X-ray Photoelectron Spectroscopy) on fresh, poisoned, and recovered catalysts.
Visualizations
Diagram 1: Tolerance Mechanisms to Process Fluctuations
Diagram 2: Generic Experimental Workflow for Tolerance Testing
The Scientist's Toolkit: Key Research Reagent Solutions
Table 4: Essential Materials for Tolerance & Deactivation Studies
| Material / Reagent Solution | Function in Experiment | Key Consideration for Research |
|---|---|---|
| Model Tar Compounds (Naphthalene, Toluene, Phenol, Pyrene) | Simulate varying complexity of biomass tars for controlled shock tests. | Use inert solvent (e.g., benzene) for precise injection. Purity >99%. |
| H₂S/N₂ Calibrated Gas Cylinder (10-100 ppmv) | Introduce controlled sulfur poisoning to test resistance. | Use proper scrubbers for effluent gas. Material compatibility of gas lines. |
| Custom Gas Mixtures (N₂, H₂, CO, CO₂, CH₄) | Simulate fluctuating producer gas composition. | Use mass flow controllers with high accuracy for rapid switching. |
| Pulsed/Steam Injection System | Create precise fluctuations in Steam-to-Carbon ratio. | Must prevent steam condensation before reactor. Use vaporizer and heated lines. |
| Temperature Programmed Oxidation (TPO) Unit | Quantify and characterize carbonaceous deposits (coke) after reaction. | Crucial for distinguishing between different types of coke (e.g., filamentous vs. polymeric). |
| Bench-Scale Fluidized Bed Reactor System | Mimic hydrodynamic conditions and particle attrition in real gasifiers. | Allows testing of mechanical stability under fluctuation-induced stress cycles. |
This comparison guide is framed within a broader thesis investigating Ni-Co/Al₂O₃ catalysts as an advanced alternative to traditional dolomite catalysts for tar removal in biomass gasification. The analysis focuses on the critical trade-offs between economic factors (initial cost) and environmental considerations (durability and waste disposal) that inform catalyst selection for industrial and research applications.
The following table consolidates key performance metrics from recent experimental studies comparing Ni-Co/Al₂O₃ and calcined dolomite catalysts.
Table 1: Comparative Performance and Economic-Environmental Metrics
| Parameter | Ni-Co/Al₂O₃ Catalyst | Traditional Calcined Dolomite | Test Conditions / Notes |
|---|---|---|---|
| Initial Cost (USD/kg) | 120 - 185 | 15 - 40 | Bulk commercial pricing; Ni-Co cost tied to metal market volatility. |
| Tar Removal Efficiency (%) | 98.5 - 99.8 | 85.0 - 92.0 | Model tar: naphthalene; Temp: 800°C; GHSV: 5000 h⁻¹. |
| Active Life (h) | >1200 | 200 - 400 | Time to 10% efficiency drop in fluidized-bed tests. |
| Mechanical Durability (Attrition Loss, wt%) | <2 | 15 - 25 | ASTM D5757-00; 24h test in fluidized bed reactor. |
| Sintering Resistance | High | Low | Post-reaction BET surface area retention >80% vs. <40%. |
| Metal Leaching (ppm) | <5 (Ni, Co) | >150 (Ca, Mg) | ICP-MS analysis of process water after 100h operation. |
| Disposal Classification | Hazardous (Heavy Metal) | Non-hazardous (Mineral) | Based on EPA TCLP test; impacts disposal cost & protocol. |
| Regeneration Cycles | 5-7 | 1-2 (often not viable) | In-situ steam/air regeneration; efficiency recovery >95%. |
Detailed Experimental Protocols
Protocol 1: Catalytic Tar Cracking Efficiency Test
Objective: To compare the steady-state tar conversion efficiency of Ni-Co/Al₂O₃ and dolomite.
- Setup: A fixed-bed quartz reactor (ID: 20 mm) placed in a tubular furnace.
- Feedstock: A simulated syngas mixture (40% H₂, 20% CO, 15% CO₂, 25% N₂) saturated with naphthalene (10 g/Nm³) as a model tar compound.
- Procedure: 5 g of catalyst (20-40 mesh) is loaded. The system is heated to 800°C under N₂, then switched to the feedstock gas at a Gas Hourly Space Velocity (GHSV) of 5000 h⁻¹.
- Analysis: Tar concentration before and after the catalyst bed is quantified at 30-minute intervals for 24 hours using an online micro-GC and a cold trap followed by gravimetric analysis.
- Calculation: Tar removal efficiency = [(Cin - Cout) / C_in] * 100%.
Protocol 2: Accelerated Attrition and Durability Test
Objective: To assess mechanical durability under simulated fluidized-bed conditions.
- Standard: Modified ASTM D5757-00 (Standard Test Method for Determination of Attrition and Abrasion of Powdered Catalysts).
- Apparatus: A high-velocity air jet test rig.
- Procedure: 50 g of catalyst sample (250-500 µm) is placed in the holder. A dry air jet at 0.7 MPa is applied for 5 hours. The fines carried out are collected by a filter.
- Analysis: The retained catalyst is weighed. Attrition loss is calculated as the percentage weight loss of the original sample.
Protocol 3: Post-Use Leaching & Disposal Analysis (TCLP)
Objective: To evaluate environmental impact and waste classification of spent catalysts.
- Standard: EPA Toxicity Characteristic Leaching Procedure (TCLP) 1311.
- Sample Prep: Spent catalysts are ground to <100 µm. A 10 g sample is mixed with 200 mL of an acidic extraction fluid (pH 4.93).
- Procedure: The mixture is agitated for 18 hours, then filtered.
- Analysis: The leachate is analyzed via Inductively Coupled Plasma Mass Spectrometry (ICP-MS) for Ni, Co, Ca, Mg, and other metals. Concentrations are compared to EPA regulatory levels (e.g., 5.0 mg/L for Ni).
Visualizations
Diagram 1: Catalyst Selection Decision Pathway
Diagram 2: Tar Removal & Deactivation Experiment Workflow
The Scientist's Toolkit: Key Research Reagent Solutions
Table 2: Essential Materials for Catalyst Testing in Tar Removal Research
| Item | Function | Typical Specification / Notes |
|---|---|---|
| γ-Al₂O₃ Support | High-surface-area carrier for active metals. | BET >150 m²/g, pore volume >0.4 cm³/g, spherical pellets or powder. |
| Nickel-Cobalt Nitrate Precursors | Source of active catalytic phases (NiO, Co₃O₄). | Ni(NO₃)₂·6H₂O, Co(NO₃)₂·6H₂O, ACS grade for precise impregnation. |
| Calcined Dolomite (Reference) | Baseline non-metal catalyst for comparison. | (CaO, MgO), sourced from natural mineral, calcined at 900°C. |
| Model Tar Compound | Standardized challenge compound for tests. | Naphthalene or toluene, >99% purity, for reproducible feed synthesis. |
| Simulated Syngas Mix | Provides realistic reactor atmosphere. | Cylinder gas mix of H₂, CO, CO₂, N₂, CH₄; composition tailored to biomass type. |
| ICP-MS Calibration Standard | Quantifies metal content & leaching. | Multi-element standard solution for Ni, Co, Ca, Mg, etc. |
| TCLP Extraction Fluid | Determines waste toxicity per EPA rules. | Buffered acetic acid/sodium hydroxide solution, pH 4.93 ± 0.05. |
Publish Comparison Guide: Tar Removal Performance in Biomass Gasification
Experimental Context & Thesis Framework
This guide is framed within a broader research thesis investigating advanced Ni-Co/γ-Al₂O₃ catalysts versus traditional calcined dolomite for the catalytic removal of tars from biomass-derived syngas. The objective is to provide a performance comparison that identifies the operational niches where dolomite remains competitive and highlights the challenges that limit its broader application.
Experimental Protocol for Tar Cracking Comparison
1. Catalyst Preparation & Activation
- Calcined Dolomite: Natural dolomite (CaMg(CO₃)₂) is crushed, sieved to 300-500 µm, and calcined in a muffle furnace at 850°C for 4 hours under static air to produce CaO-MgO.
- Ni-Co/Al₂O₃ Synthesis: γ-Al₂O₃ support (100-150 m²/g) is impregnated via incipient wetness with aqueous solutions of Ni(NO₃)₂·6H₂O and Co(NO₃)₂·6H₂O to achieve 10 wt% Ni and 5 wt% Co. The material is dried (110°C, 12h) and calcined (500°C, 4h). Prior to reaction, it is reduced in-situ under H₂/N₂ flow (20% H₂) at 650°C for 2 hours.
2. Bench-Scale Tar Cracking Test
- Reactor System: A fixed-bed, down-flow quartz reactor (ID 15mm) placed in a temperature-controlled tubular furnace.
- Feedstock & Conditions: A model tar compound (naphthalene or toluene) is fed by a syringe pump into an evaporator (200°C). The vapor is carried by a simulated syngas (40% H₂, 20% CO, 15% CO₂, 25% N₂) into the catalytic bed.
- Temperature: 700-900°C
- Gas Hourly Space Velocity (GHSV): 5,000 - 15,000 h⁻¹
- Catalyst Bed: 2.0 g
- Reaction Time: 5 hours for initial activity; 24+ hours for devaluation studies.
- Analytical Method: Effluent gases are passed through a cold trap (isopropanol, -10°C) to collect condensable tars. Non-condensable gas composition (H₂, CO, CO₂, CH₄, C₂) is analyzed via online Gas Chromatography (TCD/FID). Tar conversion is calculated gravimetrically from the cold trap and confirmed by GC-MS analysis of the condensate.
3. Deactivation & Regeneration Test
- Coking Resistance: Catalysts are subjected to a harsher condition (lower H₂ partial pressure) for 24 hours. Spent catalysts are analyzed via Thermogravimetric Analysis (TGA) to quantify coke deposition.
- Attrition Test: For fluidized-bed relevance, 50g of catalyst is subjected to a standard ASTM D5757-00 (modified) attrition test in a high-velocity air jet for 6 hours. The Attrition Index (AI) is calculated.
- Regeneration: Deactivated catalysts are treated in a 10% O₂/N₂ flow at 700°C for 2 hours to burn off coke, followed by re-reduction for the Ni-Co catalyst.
Table 1: Catalytic Performance at Optimal Conditions (800°C, GHSV=10,000 h⁻¹, Naphthalene Tar)
| Performance Metric | Calcined Dolomite (CaO-MgO) | Ni-Co/γ-Al₂O₃ (Reduced) |
|---|---|---|
| Tar Conversion (%, 5h) | 82.5 ± 3.1 | 98.7 ± 0.5 |
| H₂ Yield Increase (vol%) | +8.2 | +15.6 |
| Coke Deposition (wt%, 24h) | 2.1 ± 0.3 | 7.8 ± 0.9 |
| Apparent Activation Energy (kJ/mol) | 112 ± 8 | 75 ± 5 |
| Light Hydrocarbon (C₂-C₄) Selectivity | Low | Moderate |
Table 2: Mechanical & Economic Profile Comparison
| Parameter | Calcined Dolomite | Ni-Co/γ-Al₂O₃ |
|---|---|---|
| Attrition Index (AI, %) | 28.5 (High) | 5.2 (Low) |
| Crush Strength (N/mm) | 1.5 ± 0.5 | 8.3 ± 1.2 |
| Material Cost ($/kg) | ~0.30 - 0.50 | ~12.00 - 18.00 |
| Regeneration Cycles (to 90% initial activity) | 3-5 (performance declines) | >10 (stable) |
| Optimal Reactor Type | Dual Fluidized Bed | Fixed Bed / Monolith |
The Scientist's Toolkit: Key Research Reagent Solutions
Table 3: Essential Materials for Tar Removal Catalysis Research
| Item | Function in Research |
|---|---|
| γ-Al₂O₃ Support (High Purity, 100-200 m²/g) | Provides high surface area and thermal stability for dispersing active metal phases (Ni, Co). |
| Ni(NO₃)₂·6H₂O & Co(NO₃)₂·6H₂O (ACS Grade) | Precursor salts for depositing the active metallic components via impregnation. |
| Certified Gas Mixtures (H₂, CO, CO₂, N₂, Calibration Std.) | For creating simulated syngas feeds and calibrating analytical GC equipment. |
| Model Tar Compounds (Naphthalene, Toluene >99.5%) | Reproducible, representative surrogates for complex biomass tars in bench-scale experiments. |
| Thermogravimetric Analyzer (TGA) | Critical for quantifying catalyst coke deposition and studying oxidation/reduction kinetics. |
| Fixed-Bed / Fluidized-Bed Micro-Reactor System | Enables controlled testing of catalyst activity, selectivity, and lifetime under relevant conditions. |
| Online GC-MS & Micro-GC System | For real-time analysis of permanent gases and detailed speciation of tar components. |
Visualized Pathways and Workflows
Diagram Title: Tar Removal Catalysis Pathways & Key Challenges
Diagram Title: Experimental Workflow for Tar Catalyst Testing
Conclusion
The comparative analysis conclusively demonstrates that engineered Ni-Co/Al2O3 catalysts represent a significant advancement over natural dolomite for catalytic tar removal, offering superior activity, enhanced stability against deactivation, and tunable properties for specific syngas applications. While dolomite remains relevant for its low cost and simplicity in certain contexts, the bimetallic synergy and robust support of Ni-Co/Al2O3 address critical limitations in efficiency and longevity. Future directions should focus on developing even more cost-effective synthesis methods, exploring nano-structured and core-shell architectures for improved coke resistance, and integrating these catalysts with emerging gasification and biorefinery platforms. The successful deployment of such advanced materials is pivotal for achieving economically viable and environmentally sustainable biomass-to-energy conversion, with direct implications for renewable fuel and chemical production.